Asparaginyl endopeptidases: enzymology, applications and limitations
- PMID: 34037066
- PMCID: PMC8209628
- DOI: 10.1039/d1ob00608h
Asparaginyl endopeptidases: enzymology, applications and limitations
Abstract
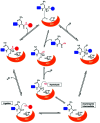
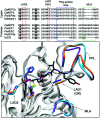
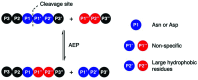
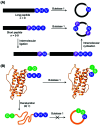
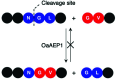
Asparaginyl endopeptidases (AEP) are cysteine proteases found in mammalian and plant cells. Several AEP isoforms from plant species were found to exhibit transpeptidase activity which is integral for the key head-to-tail cyclisation reaction during the biosynthesis of cyclotides. Since many plant AEPs exhibit excellent enzyme kinetics for peptide ligation via a relatively short substrate recognition sequence, they have become appealing tools for peptide and protein modification. In this review, research focused on the enzymology of AEPs and their applications in polypeptide cyclisation and labelling will be presented. Importantly, the limitations of using AEPs and opportunities for future research and innovation will also be discussed.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Macrocyclization by asparaginyl endopeptidases.New Phytol. 2018 May;218(3):923-928. doi: 10.1111/nph.14511. Epub 2017 Mar 21. New Phytol. 2018. PMID: 28322452 Review.
-
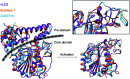
Molecular basis for the production of cyclic peptides by plant asparaginyl endopeptidases.Nat Commun. 2018 Jun 20;9(1):2411. doi: 10.1038/s41467-018-04669-9. Nat Commun. 2018. PMID: 29925835 Free PMC article.
-
Make it or break it: Plant AEPs on stage in biotechnology.Biotechnol Adv. 2020 Dec;45:107651. doi: 10.1016/j.biotechadv.2020.107651. Epub 2020 Oct 23. Biotechnol Adv. 2020. PMID: 33141031 Review.
-
A suite of kinetically superior AEP ligases can cyclise an intrinsically disordered protein.Sci Rep. 2019 Jul 25;9(1):10820. doi: 10.1038/s41598-019-47273-7. Sci Rep. 2019. PMID: 31346249 Free PMC article.
-
In Vitro and In Planta Cyclization of Target Peptides Using an Asparaginyl Endopeptidase from Oldenlandia affinis.Methods Mol Biol. 2019;2012:211-235. doi: 10.1007/978-1-4939-9546-2_12. Methods Mol Biol. 2019. PMID: 31161511
Cited by
-
Intracellular Application of an Asparaginyl Endopeptidase for Producing Recombinant Head-to-Tail Cyclic Proteins.JACS Au. 2023 Nov 20;3(12):3290-3296. doi: 10.1021/jacsau.3c00591. eCollection 2023 Dec 25. JACS Au. 2023. PMID: 38155637 Free PMC article.
-
Genetic Code Expansion Approaches to Decipher the Ubiquitin Code.Chem Rev. 2024 Oct 23;124(20):11544-11584. doi: 10.1021/acs.chemrev.4c00375. Epub 2024 Sep 23. Chem Rev. 2024. PMID: 39311880 Free PMC article. Review.
-
Enzymatic Spin-Labeling of Protein N- and C-Termini for Electron Paramagnetic Resonance Spectroscopy.Bioconjug Chem. 2023 Mar 15:10.1021/acs.bioconjchem.3c00029. doi: 10.1021/acs.bioconjchem.3c00029. Online ahead of print. Bioconjug Chem. 2023. PMID: 36921260 Free PMC article.
-
Novel Haloacethydrazides as AEP Inhibitors for Treating Alzheimer's Disease.ACS Med Chem Lett. 2023 Sep 5;14(9):1165-1166. doi: 10.1021/acsmedchemlett.3c00367. eCollection 2023 Sep 14. ACS Med Chem Lett. 2023. PMID: 37736172 Free PMC article.
-
Towards controlling activity of a peptide asparaginyl ligase (PAL) by lumazine synthetase compartmentalization.Faraday Discuss. 2024 Sep 11;252(0):403-421. doi: 10.1039/d4fd00002a. Faraday Discuss. 2024. PMID: 38832470 Free PMC article.
References
-
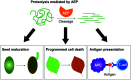
- Dall E. Brandstetter H. Structure and function of legumain in health and disease. Biochimie. 2016;122:126–150. - PubMed
-
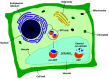
- Hayashi Y. Yamada K. Shimada T. Matsushima R. Nishizawa N. K. Nishimura M. Hara-Nishimura I. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol. 2001;42:894–899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

