Specific Phospholipid Modulation by Muscarinic Signaling in a Rat Lesion Model of Alzheimer's Disease
- PMID: 34037379
- PMCID: PMC9162383
- DOI: 10.1021/acschemneuro.1c00169
Specific Phospholipid Modulation by Muscarinic Signaling in a Rat Lesion Model of Alzheimer's Disease
Abstract
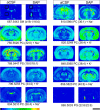
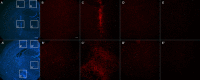
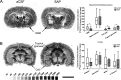
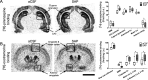
Alzheimer's disease (AD) represents the most common cause of dementia worldwide and has been consistently associated with the loss of basal forebrain cholinergic neurons (BFCNs) leading to impaired cholinergic neurotransmission, aberrant synaptic function, and altered structural lipid metabolism. In this sense, membrane phospholipids (PLs) can be used for de novo synthesis of choline (Ch) for the further obtaining of acetylcholine (ACh) when its availability is compromised. Specific lipid species involved in the metabolism of Ch have been identified as possible biomarkers of phenoconversion to AD. Using a rat model of BFCN lesion, we have evaluated the lipid composition and muscarinic signaling in brain areas related to cognitive processes. The loss of BFCN resulted in alterations of varied lipid species related to Ch metabolism at nucleus basalis magnocellularis (NMB) and cortical projection areas. The activity of muscarinic receptors (mAChR) was decreased in the NMB and increased in the hippocampus according to the subcellular distribution of M1/M2 mAChR which could explain the learning and memory impairment reported in this AD rat model. These results suggest that the modulation of specific lipid metabolic routes could represent an alternative therapeutic strategy to potentiate cholinergic neurotransmission and preserve cell membrane integrity in AD.
Keywords: 192IgG-saporin; Cholinergic; MALDI; autoradiography; lipid; muscarinic receptor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Perry E. K.; Gibson P. H.; Blessed G.; Perry R. H.; Tomlinson B. E. (1977) Neurotransmitter Enzyme Abnormalities in Senile Dementia. Choline Acetyltransferase and Glutamic Acid Decarboxylase Activities in Necropsy Brain Tissue. J. Neurol. Sci. 34 (2), 247–265. 10.1016/0022-510X(77)90073-9. - DOI - PubMed
-
- Rodríguez-Puertas R.; Pazos A.; Zarranz J. J.; Pascual J. (1994) Selective Cortical Decrease of High-Affinity Choline Uptake Carrier in Alzheimer’s Disease: An Autoradiographic Study Using 3H-Hemicholinium-3. J. Neural Transm.: Parkinson's Dis. Dementia Sect. 8 (3), 161–169. 10.1007/BF02260937. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

