SAMM50 acts with p62 in piecemeal basal- and OXPHOS-induced mitophagy of SAM and MICOS components
- PMID: 34037656
- PMCID: PMC8160579
- DOI: 10.1083/jcb.202009092
SAMM50 acts with p62 in piecemeal basal- and OXPHOS-induced mitophagy of SAM and MICOS components
Abstract
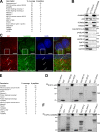
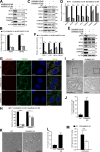
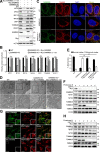
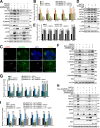
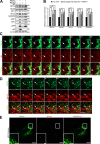
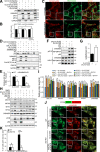
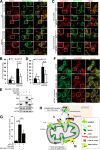
Mitophagy is the degradation of surplus or damaged mitochondria by autophagy. In addition to programmed and stress-induced mitophagy, basal mitophagy processes exert organelle quality control. Here, we show that the sorting and assembly machinery (SAM) complex protein SAMM50 interacts directly with ATG8 family proteins and p62/SQSTM1 to act as a receptor for a basal mitophagy of components of the SAM and mitochondrial contact site and cristae organizing system (MICOS) complexes. SAMM50 regulates mitochondrial architecture by controlling formation and assembly of the MICOS complex decisive for normal cristae morphology and exerts quality control of MICOS components. To this end, SAMM50 recruits ATG8 family proteins through a canonical LIR motif and interacts with p62/SQSTM1 to mediate basal mitophagy of SAM and MICOS components. Upon metabolic switch to oxidative phosphorylation, SAMM50 and p62 cooperate to mediate efficient mitophagy.
© 2021 Abudu et al.
Figures







References
-
- Adams, P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W., et al. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

