Neuroendocrine Basis for Disrupted Ovarian Cyclicity in Female Mice During Chronic Undernutrition
- PMID: 34037744
- PMCID: PMC8272537
- DOI: 10.1210/endocr/bqab103
Neuroendocrine Basis for Disrupted Ovarian Cyclicity in Female Mice During Chronic Undernutrition
Abstract
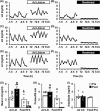
Chronic undernutrition is a type of metabolic stress that impairs reproduction in multiple species. Although energy balance and female reproductive capacity is recognized as tightly coupled, the neuroendocrine loci and molecular mechanisms that mediate ovarian cycle dysfunction during chronic undernutrition in adult females remain poorly understood. Here, we present a series of studies in which we tested the hypothesis that inhibition of kisspeptin (Kiss1) neurons, which are critical for controlling luteinizing hormone (LH) pulses and the preovulatory LH surge in females, underlies the impairment of the ovarian cycle by undernutrition. We first investigated the effect of chronic undernutrition (70% of unrestricted feed intake) on estrous cyclicity in intact female c57bl6 mice. Undernutrition caused a rapid cessation of ovarian cyclicity during the 2-week treatment, suppressing ovarian steroidogenesis and inhibiting ovulation. Using 2 well-defined estradiol-replacement paradigms, we directly tested the hypothesis that undernutrition inhibits Kiss1 neurons in the arcuate nucleus (ARCKiss1), which are required for LH pulses and in the anteroventral periventricular nucleus (AVPVKiss1), which are necessary for LH surge secretion. Undernutrition prevented LH pulses and impaired ARCKiss1 neuronal activation, using c-Fos as a marker, in ovariectomized females subcutaneously implanted with a pellet containing a diestrus-like level of estradiol. In addition, undernutrition completely blocked the estradiol-induced LH surge and diminished Kiss1 messenger RNA abundance, without decreasing estradiol receptor α (Erα), in micropunches of the AVPV. Collectively, these studies demonstrate that undernutrition disrupts ovarian cyclicity in females via impairment both of ARCKiss1 control of LH pulses and AVPVKiss1 induction of the LH surge.
Keywords: feed restriction; gonadotropin-releasing hormone; kisspeptin; luteinizing hormone; metabolism; stress.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures







References
-
- Bronson FH, Marsteller FA. Effect of short-term food deprivation on reproduction in female mice. Biol Reprod. 1985;33(3):660-667. - PubMed
-
- Foster DL, Ebling FJ, Micka AF, et al. Metabolic interfaces between growth and reproduction. I. Nutritional modulation of gonadotropin, prolactin, and growth hormone secretion in the growth-limited female lamb. Endocrinology. 1989;125(1):342-350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

