Testing the feasibility of operationalizing a prospective, randomized trial with remote cardiac safety EKG monitoring during a pandemic
- PMID: 34037911
- PMCID: PMC8149293
- DOI: 10.1007/s10840-021-00989-x
Testing the feasibility of operationalizing a prospective, randomized trial with remote cardiac safety EKG monitoring during a pandemic
Abstract
Background: The coronavirus SARS-CoV-2 is highly contagious. Hydroxychloroquine (HCQ) has in vitro activity against SARS-CoV-2. The FDA authorized emergency use of HCQ against COVID-19. HCQ may have dose-related cardiotoxicity. This clinical trial received ethical approval on May 15, 2020, operationalized in June to evaluate a low prophylaxis dose of HCQ (200mg BID) in household contacts of COVID-19-positive patients without physical contact between investigators and participants. It represents the first report of the FDA approved 6-lead EKGs with a smartphone KardiaMobile® 6L application.
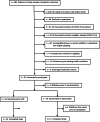
Methods: To reach a sample size of 170, household members were contacted by telephone, emailed consent forms with electronic signature capability, and randomized 2:1 to HCQ or observation for 10 days with follow-up of 14 days. Home saliva PCR tests recorded COVID status on days 1 and 14. Symptoms and 6-lead EKGs were obtained daily.
Results: Fifty-one participants were randomized with 42 evaluable at day 14. Remote monitoring of 407 EKGs revealed no QTc prolongation or other ECG changes in either group. At time of consent, no participants were symptomatic or COVID+. On days 1 and 14, COVID tests were positive in 4 and 2 in the HCQ group and 4 and 0 in the observation group. No tests converted to positive. There were no deaths or hospitalizations.
Conclusions: A clinical trial without personal contact, rapidly initiated and operationalized to exclude cardiac toxicity using daily remote 6-lead EKG monitoring, is feasible. Of 407 EKGs from 42 participants, there was no evidence of cardiac toxicity.
Clinical trial registration: Clinicaltrials.gov : NCT04652648 registration date: December 3, 2020.
Keywords: COVID-19; EKG monitoring; Hydroxychloroquine; Prospective trial; SARS-CoV-2.
© 2021. Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

