Dupilumab suppresses type 2 inflammatory biomarkers across multiple atopic, allergic diseases
- PMID: 34037993
- PMCID: PMC8362102
- DOI: 10.1111/cea.13954
Dupilumab suppresses type 2 inflammatory biomarkers across multiple atopic, allergic diseases
Abstract
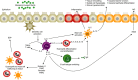
Background: Type 2 inflammation is common in numerous atopic/allergic diseases and can be identified by elevated biomarker levels. Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and interleukin-13, key and central drivers of type 2 inflammation.
Objective: Assessment of dupilumab effect on type 2 inflammatory biomarkers in atopic dermatitis (AD), asthma, chronic rhinosinusitis with nasal polyps (CRSwNP) and eosinophilic esophagitis (EoE).
Methods: Data were extracted from three randomized placebo-controlled trials of dupilumab in AD (NCT02277743, N = 671; NCT02277769, N = 708; NCT02260986, N = 740); and one each in asthma (NCT02414854, N = 1902); CRSwNP (NCT02898454, N = 448); and EoE (NCT02379052, N = 47). Biomarkers assessed were serum thymus and activation-regulated chemokine (TARC), plasma eotaxin-3, serum total immunoglobulin E (IgE), serum periostin and blood eosinophil count.
Results: Dupilumab versus placebo significantly suppressed most type 2 inflammatory biomarker levels across all studies/indications where data were assessed. Reductions in serum TARC, plasma eotaxin-3 and serum periostin occurred rapidly, whereas reductions in serum total IgE were more gradual. Across diseases, at the end of treatment, median percentage change from baseline in TARC levels ranged from -24.8% to -88.6% (placebo +2.6% to -53.6%); -38.2% to -51.5% (placebo +8.3% to -0.16%) in eotaxin-3; -24.8% to -76.7% (placebo +8.3% to -4.4%) in total IgE; and -13.6% to -41.1% (placebo +10.1% to -6.94%) in periostin levels. Blood eosinophil responses to dupilumab varied by disease, with minimal changes in AD in the SOLO studies (median percentage change from baseline to end of treatment: 0% [95% CI: -15.8, 0]); transient increases followed by decreases to below-baseline levels in asthma (-14.6% [-20.0, -7.7]) and CRSwNP (-29.4% [-40.0, -16.3]); and significant decreases in EoE (-50.0% [-50.0, -33.3]).
Conclusion and clinical relevance: Dupilumab reduced levels of type 2 biomarkers across clinical studies in patients with AD, asthma, CRSwNP and EoE.
Keywords: asthma; atopic dermatitis; chronic rhinosinusitis with nasal polyposis; dupilumab; eosinophilic esophagitis.
© 2021 The Authors. Clinical & Experimental Allergy published by John Wiley & Sons Ltd.
Conflict of interest statement
JDH, SH, ZC, NA, MA, AR, BS and MR are employees and shareholders of Regeneron Pharmaceuticals, Inc. NMHG is a prior employee and shareholder of Regeneron Pharmaceuticals, Inc. WB, MSR, NP and LM are employees and may hold stock and/or stock options in Sanofi. BG and GP are prior employees and may hold stock and/or stock options in Sanofi.
Figures





References
-
- Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425‐437. - PubMed
-
- Robinson D, Humbert M, Buhl R, et al. Revisiting type 2‐high and type 2‐low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47(2):161‐175. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

