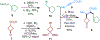Total Synthesis of Penicibilaenes via C-C Activation-Enabled Skeleton Deconstruction and Desaturation Relay-Mediated C-H Functionalization
- PMID: 34038107
- PMCID: PMC9112325
- DOI: 10.1021/jacs.1c04335
Total Synthesis of Penicibilaenes via C-C Activation-Enabled Skeleton Deconstruction and Desaturation Relay-Mediated C-H Functionalization
Abstract
Herein, we describe the first total synthesis of sesquiterpene penicibilaenes A and B through a "C-C/C-H" approach. In the "C-C" stage, the Rh-catalyzed "cut-and-sew" transformation between trisubstituted alkene and cyclobutanone has been employed to construct the unique tricyclo[6.3.1.01,5]dodecane skeleton and the all-carbon quaternary center. Critical linker and Lewis acid effects have been identified for the C-C activation process. In the "C-H" stage, a desaturation relay-based strategy involving consecutive ketone α,β-dehydrogenation and β-functionalization has been adopted to introduce the 1,3,5-triad stereocenters to the core. The synthesis of penicibilaenes A and B has been completed in 13 and 14 steps, respectively, in the longest linear sequence.
Figures
References
-
- Breitmaier E Terpenes : Flavors, Fragrances, Pharmaca, Pheromones; WILEY-VCH: Weinheim, 2006.
-
- Cherney EC; Baran PS Terpenoid-Alkaloids: Their Biosynthetic Twist of Fate and Total Synthesis. Isr. J. Chem 2011, 51, 391–405. - PMC - PubMed
- Justicia J; de Cienfuegos LA; Campana AG; Miguel D; Jakoby V; Gansauer A; Cuerva JM Bioinspired Terpene Synthesis: a Radical Approach. Chem. Soc. Rev 2011, 40, 3525–3537. - PubMed
- Urabe D; Asaba T; Inoue M Convergent Strategies in Total Syntheses of Complex Terpenoids. Chem. Rev 2015, 115, 9207–9231. - PubMed
- Brill ZG; Condakes ML; Ting CP; Maimone TJ Navigating the Chiral Pool in the Total Synthesis of Complex Terpene Natural Products. Chem. Rev 2017, 117, 11753–11795. - PMC - PubMed
-
- For selected examples and reviews, see: Chen K; Baran PS Total Synthesis of Eudesmane Terpenes by Site-Selective C─H Oxidations. Nature 2009, 459, 824–828. - PubMed
- Ishihara Y; Baran PS Two-Phase Terpene Total Synthesis: Historical Perspective and Application to the Taxol® Problem. Synlett 2010, 1733–1745.
- Foo K; Usui I; Gotz DCG; Werner EW; Holte D; Baran PS Scalable, Enantioselective Synthesis of Germacrenes and Related Sesquiterpenes Inspired by Terpene Cyclase Phase Logic. Angew. Chem., Int. Ed 2012, 51, 11491–11495. - PMC - PubMed
- Jorgensen L; McKerrall SJ; Kuttruff CA; Ungeheuer F; Felding J; Baran PS 14-Step Synthesis of (+)-Ingenol from (+)-3-Carene. Science 2013, 341, 878–882. - PubMed
- Wilde NC; Isomura M; Mendoza A; Baran PS Two-Phase Synthesis of (−)-Taxuyunnanine D. J. Am. Chem. Soc 2014, 136, 4909–4912. - PMC - PubMed
- Kanda Y; Nakamura H; Umemiya S; Puthukanoori RK; Appala VRM; Gaddamanugu GK; Paraselli BR; Baran PS Two-Phase Synthesis of Taxol. J. Am. Chem. Soc 2020, 142, 10526–10533. - PMC - PubMed
-
- Jun CH Transition Metal-Catalyzed Carbon–Carbon Bond Activation. Chem. Soc. Rev 2004, 33, 610–618. - PubMed
- Dong G Ed. C─C Bond Activation; Springer-Verlag: Berlin, 2014; Vol. 346.
- Souillart L; Cramer N Catalytic C─C Bond Activations via Oxidative Addition to Transition Metals. Chem. Rev 2015, 115, 9410–9464. - PubMed
- Fumagalli G; Stanton S; Bower JF Recent Methodologies That Exploit C─C Single-Bond Cleavage of Strained Ring Systems by Transition Metal Complexes. Chem. Rev 2017, 117, 9404–9432. - PubMed
- Deng L; Dong G Carbon─Carbon Bond Activation of Ketones. Trends Chem. 2020, 2, 183–198.
- Murakami M; Ishida N Cleavage of Carbon–Carbon σ-Bonds of Four-Membered Rings. Chem. Rev 2021, 121, 264–299. - PubMed
-
- Xu T; Dong G Rhodium-Catalyzed Regioselective Carboacylation of Olefins: A C─C Bond Activation Approach for Accessing Fused-Ring Systems. Angew. Chem., Int. Ed 2012, 51, 7567–7571. - PubMed
- Chen PH; Billett BA; Tsukamoto T; Dong G "Cut and Sew" Transformations via Transition-Metal-Catalyzed Carbon─Carbon Bond Activation. ACS Catal. 2017, 7, 1340–1360. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources