Launching a saliva-based SARS-CoV-2 surveillance testing program on a university campus
- PMID: 34038425
- PMCID: PMC8153421
- DOI: 10.1371/journal.pone.0251296
Launching a saliva-based SARS-CoV-2 surveillance testing program on a university campus
Abstract
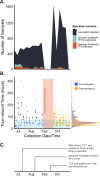
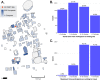
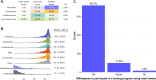
Regular surveillance testing of asymptomatic individuals for SARS-CoV-2 has been center to SARS-CoV-2 outbreak prevention on college and university campuses. Here we describe the voluntary saliva testing program instituted at the University of California, Berkeley during an early period of the SARS-CoV-2 pandemic in 2020. The program was administered as a research study ahead of clinical implementation, enabling us to launch surveillance testing while continuing to optimize the assay. Results of both the testing protocol itself and the study participants' experience show how the program succeeded in providing routine, robust testing capable of contributing to outbreak prevention within a campus community and offer strategies for encouraging participation and a sense of civic responsibility.
Conflict of interest statement
The authors have read the journal’s policy and the authors of this manuscript have the following competing interests: The Regents of the University of California have patents issued and pending for CRISPR technologies on which JAD is an inventor. JAD is a co-founder of Caribou Biosciences, Editas Medicine, Scribe Therapeutics, Intellia Therapeutics, and Mammoth Biosciences. JAD is a scientific advisory board member of Caribou Biosciences, Intellia Therapeutics, eFFECTOR Therapeutics, Scribe Therapeutics, Mammoth Biosciences, Synthego, Algen Biotechnologies, Felix Biosciences, and Inari. JAD is a Director at Johnson & Johnson and Tempus Labs and has research projects sponsored by Biogen, Pfizer, AppleTree Partners, and Roche. FDU is a co-founder of Tune Therapeutics. PG is a co-founder and Director at NewCo Health. PG is the CLIA Laboratory Director for Coral Genomics and 3DMed. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
-
- Chitwood MH, Russi M, Gunasekera K, Havumaki J, Pitzer VE, Warren JL, et al.. Bayesian nowcasting with adjustment for delayed and incomplete reporting to estimate COVID-19 infections in the United States. medRxiv. 2020:2020.06.17.20133983.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

