Candesartan as a tentative treatment for COVID-19: A prospective non-randomized open-label study
- PMID: 34038766
- PMCID: PMC8142270
- DOI: 10.1016/j.ijid.2021.05.019
Candesartan as a tentative treatment for COVID-19: A prospective non-randomized open-label study
Abstract
Background: This study aimed to investigate whether the addition of candesartan to the standard care regimen improved the outcome in patients with coronavirus 2019 (COVID-19).
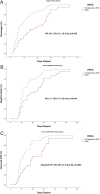
Methods: A prospective non-randomized open-label study was undertaken from May to August 2020 on 75 subjects (aged 18-70 years) hospitalized in Siloam Kelapa Dua Hospital. Uni- and multi-variable Cox regression analyses were performed to obtain hazard ratios (HRs). The primary outcomes were: (1) length of hospital stay; (2) time to negative swab; and (3) radiological outcome (time to improvement on chest X ray).
Results: None of the 75 patients with COVID-19 required intensive care. All patients were angiotensin-receptor-blocker naïve. In comparison with the control group, the candesartan group had a significantly shorter hospital stay [adjusted HR 2.47, 95% confidence interval (CI) 1.16-5.29] after adjusting for a wide range of confounders, and no increased risk of intensive care. In the non-obese subgroup, the candesartan group had a shorter time to negative swab (unadjusted HR 2.11, 95% CI 1.02-4.36; adjusted HR 2.40, 95% CI 1.08-5.09) and shorter time to improvement in chest x ray (adjusted HR 2.82, 95% CI 1.13-7.03) compared with the control group.
Conclusion: Candesartan significantly reduces the length of hospital stay after adjustment for covariates. All primary outcomes improved significantly in the non-obese subgroup receiving candesartan.
Keywords: Angiotensin; Candesartan; Coronavirus; SARS-CoV-2; Severity.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures






References
-
- Day M. COVID-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369:m1375. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

