Outcomes of patients with end stage kidney disease on dialysis with COVID-19 in Abu Dhabi, United Arab Emirates; from PCR to antibody
- PMID: 34039299
- PMCID: PMC8152185
- DOI: 10.1186/s12882-021-02378-y
Outcomes of patients with end stage kidney disease on dialysis with COVID-19 in Abu Dhabi, United Arab Emirates; from PCR to antibody
Abstract
Background: Individuals with end-stage kidney disease (ESKD) on dialysis are vulnerable to contracting COVID-19 infection, with mortality as high as 31 % in this group. Population demographics in the UAE are dissimilar to many other countries and data on antibody responses to COVID-19 is also limited. The objective of this study was to describe the characteristics of patients who developed COVID-19, the impact of the screening strategy, and to assess the antibody response to a subset of dialysis patients.
Methods: We retrospectively examined the outcomes of COVID19 infection in all our haemodialysis patients, who were tested regularly for COVID 19, whether symptomatic or asymptomatic. In addition, IgG antibody serology was also performed to assess response to COVID-19 in a subset of patients.
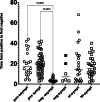
Results: 152 (13 %) of 1180 dialysis patients developed COVID-19 during the study period from 1st of March to the 1st of July 2020. Of these 81 % were male, average age of 52 years and 95 % were on in-centre haemodialysis. Family and community contact was most likely source of infection in most patients. Fever (49 %) and cough (48 %) were the most common presenting symptoms, when present. Comorbidities in infected individuals included hypertension (93 %), diabetes (49 %), ischaemic heart disease (30 %). The majority (68 %) developed mild disease, whilst 13 % required critical care. Combinations of drugs including hydroxychloroquine, favipiravir, lopinavir, ritonavir, camostat, tocilizumab and steroids were used based on local guidelines. The median time to viral clearance defined by two negative PCR tests was 15 days [IQR 6-25]. Overall mortality in our cohort was 9.2 %, but ICU mortality was 65 %. COVID-19 IgG antibody serology was performed in a subset (n = 87) but 26 % of PCR positive patients (n = 23) did not develop a significant antibody response.
Conclusions: Our study reports a lower mortality in this patient group compared with many published series. Asymptomatic PCR positivity was present in 40 %. Rapid isolation of positive patients may have contributed to the relative lack of spread of COVID-19 within our dialysis units. The lack of antibody response in a few patients is concerning.
Keywords: COVID-19; Dialysis; End stage kidney disease; IgG antibody; Mortality; Screening.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

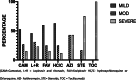
Similar articles
-
Investigating the utility of COVID-19 antibody testing in end-stage renal disease patients receiving haemodialysis: a cohort study in the United Kingdom.BMC Nephrol. 2021 Apr 27;22(1):154. doi: 10.1186/s12882-021-02366-2. BMC Nephrol. 2021. PMID: 33902482 Free PMC article.
-
Antibody response using six different serological assays in a completely PCR-tested community after a coronavirus disease 2019 outbreak-the CoNAN study.Clin Microbiol Infect. 2021 Mar;27(3):470.e1-470.e9. doi: 10.1016/j.cmi.2020.11.009. Epub 2020 Nov 20. Clin Microbiol Infect. 2021. PMID: 33221432 Free PMC article.
-
Antibody Status, Disease History, and Incidence of SARS-CoV-2 Infection Among Patients on Chronic Dialysis.J Am Soc Nephrol. 2021 Aug;32(8):1880-1886. doi: 10.1681/ASN.2021030387. Epub 2021 Jul 2. J Am Soc Nephrol. 2021. PMID: 34215666 Free PMC article.
-
Assessment and management of asymptomatic COVID-19 infection: A systematic review.Travel Med Infect Dis. 2021 May-Jun;41:102058. doi: 10.1016/j.tmaid.2021.102058. Epub 2021 Apr 7. Travel Med Infect Dis. 2021. PMID: 33838319 Free PMC article.
-
Ravaging SARS-CoV-2: rudimentary diagnosis and puzzling immunological responses.Curr Med Res Opin. 2021 Feb;37(2):207-217. doi: 10.1080/03007995.2020.1862532. Epub 2020 Dec 26. Curr Med Res Opin. 2021. PMID: 33306409 Free PMC article. Review.
Cited by
-
The Time to Negative Conversion among Adult COVID-19 Patients on Chronic Hemodialysis Admitted at the Philippine General Hospital - A Retrospective Cohort Study.Acta Med Philipp. 2024 Mar 22;58(5):22-27. doi: 10.47895/amp.vi0.6460. eCollection 2024. Acta Med Philipp. 2024. PMID: 39005616 Free PMC article.
-
An analysis of antibody responses and clinical sequalae of the Sinopharm HB02 COVID19 vaccine in dialysis patients in the United Arab Emirates.Nephrology (Carlton). 2022 Mar;27(3):260-268. doi: 10.1111/nep.13980. Epub 2021 Oct 5. Nephrology (Carlton). 2022. PMID: 34569677 Free PMC article.
-
Risk factors for mortality in hemodialysis patients with COVID-19: a systematic review and meta-analysis.Ren Fail. 2021 Dec;43(1):1394-1407. doi: 10.1080/0886022X.2021.1986408. Ren Fail. 2021. PMID: 34629011 Free PMC article.
-
Clinical manifestations of COVID-19 infection in dialysis patients and protective effect of COVID-19 vaccine.Inflamm Res. 2023 May;72(5):989-1000. doi: 10.1007/s00011-023-01723-1. Epub 2023 Apr 1. Inflamm Res. 2023. PMID: 37004547 Free PMC article. Review.
-
The Omicron COVID-19 threat to dialysis patients is dramatically lower than previous variants.Nephrology (Carlton). 2022 Aug;27(8):725-726. doi: 10.1111/nep.14065. Epub 2022 May 31. Nephrology (Carlton). 2022. PMID: 35641846 Free PMC article. No abstract available.
References
-
- He F, Deng Y, Li W. Coronavirus disease 2019: What we know? [Internet]. Vol. 92, Journal of Medical Virology. John Wiley and Sons Inc.; 2020 [cited 2020 Aug 16]. p. 719–25. Available from: https://pubmed.ncbi.nlm.nih.gov/32170865/ - PMC - PubMed
-
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA - J Am Med Assoc [Internet]. 2020 Apr 28 [cited 2020 Aug 16];323(16):1574–81. Available from: https://jamanetwork.com/ - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

