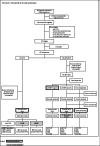
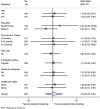
Comparison of cervical cancer screening by self-sampling papillomavirus test versus pap-smear in underprivileged women in France
- PMID: 34039341
- PMCID: PMC8157706
- DOI: 10.1186/s12905-021-01356-8
Comparison of cervical cancer screening by self-sampling papillomavirus test versus pap-smear in underprivileged women in France
Abstract
Background: The purpose of this study was to compare cervical cancer screening by pap smear (PS) versus preliminary HPV testing based on self-collected samples (SC-HPV).
Methods: Interventional study among underprivileged women from 25 to 65 years old in four French cities. The control group (CG) was referred for a PS. The experimental group (EG) conducted a SC-HPV test followed by a PS in case of positivity. Differences on screening completion and cytological abnormalities were analysed by logistic and Cox regression.
Results: 383 women were assigned to the EG and 304 to the CG. The screening completion proportion was 39.5% in the CG compared to 71.3% in the EG (HR = 2.48 (CI 95% [1.99-3.08]; p < 0.001). The proportion of cytological abnormalities was 2.0% in the CG and 2.3% in the EG (OR = 1.20 (CI 95% [0.42-3.40]; p = 0.7). The proportion of participants lost to follow-up was 60.5% in the CG and 63.2% in the EG HPV positive (p = 0.18).
Conclusion: Providing an SC-HPV-test increased the participation of underprivileged women in CCS. Nevertheless, the significant number of lost to follow-up in both groups can undermine the initial benefits of the strategy for HPV positive women. Registration: Clinicaltrials.gov: NCT03118258.
Keywords: Cervical cancer; Papillomavirus; SC-HPV; Screening; Vulnerability.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
References
-
- Centre National de Référence des Papillomavirus Humains (CNR HPV), Institut Pasteur. Référence des Papillomavirus Humains (CNR HPV), Institut Pasteur. In 2018. Disponible sur: www.pasteur.fr/fr/centre-medical/fiches-maladies/cancer-du-coluterus-pap...
-
- Garnier A, Brindel P. Prévention et dépistage du cancer du col de l’utérus. Boulogne [Internet]. Billancourt: Institut National du Cancer; 2013. Disponible sur: www.ecancer.fr/publications/75-prevention/735-prevention-et-depistage-du....
-
- Institute National du Cancer. La situation du cancer en France. 2012.
-
- Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primer. 01 2016;2:16086. - PubMed
-
- GLOBOCAN [Internet]. Disponible sur: https://onlinelibrary.wiley.com/doi/full/10.3322/caac.21492) - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials