The microbiota in cirrhosis and its role in hepatic decompensation
- PMID: 34039493
- PMCID: PMC8973011
- DOI: 10.1016/j.jhep.2020.11.013
The microbiota in cirrhosis and its role in hepatic decompensation
Abstract
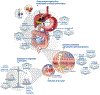
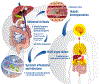
Cirrhosis - the common end-stage of chronic liver disease - is associated with a cascade of events, of which intestinal bacterial overgrowth and dysbiosis are central. Bacterial toxins entering the portal or systemic circulation can directly cause hepatocyte death, while dysbiosis also affects gut barrier function and increases bacterial translocation, leading to infections, systemic inflammation and vasodilation, which contribute to acute decompensation and organ failure. Acute decompensation and its severe forms, pre-acute-on-chronic liver failure (ACLF) and ACLF, are characterised by sudden organ dysfunction (and failure) and high short-term mortality. Patients with pre-ACLF and ACLF present with high-grade systemic inflammation, usually precipitated by proven bacterial infection and/or severe alcoholic hepatitis. However, no precipitant is identified in 30% of these patients, in whom bacterial translocation from the gut microbiota is assumed to be responsible for systemic inflammation and decompensation. Different microbiota profiles may influence the rate of decompensation and thereby outcome in these patients. Thus, targeting the microbiota is a promising strategy for the prevention and treatment of acute decompensation, pre-ACLF and ACLF. Approaches include the use of antibiotics such as rifaximin, faecal microbial transplantation and enterosorbents (e.g. Yaq-001), which bind microbial factors without exerting a direct effect on bacterial growth kinetics. This review focuses on the role of microbiota in decompensation and strategies targeting microbiota to prevent acute decompensation.
Keywords: Acute decompensation; Acute-on-chronic liver failure; Cirrhosis; Gut-liver-axis; Portal hypertension.
Copyright © 2020 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest J.T. has received speaking and/or consulting fees from Gore, Bayer, Alexion, MSD, Gilead, Intercept, Norgine, Grifols, Versantis, and Martin Pharmaceutical. J.M. is a co-founder of Yaqrit Limited and has received speaker fees from Norgine and Yakult Europe. B.S. has been consulting for Ferring Research Institute, Intercept Pharmaceuticals, HOST Therabiomics, Mabwell Therapeutics and Patara Pharmaceuticals. B.S.’s institution UC San Diego has received grant support from BiomX, NGM Biopharmaceuticals, CymaBay Therapeutics, Synlogic Operating Company and Axial Biotherapeutics. D.L.S. has been consulting for Norgine, Kaleido Biosciences, Shionogi and Mallinckrodt Pharmaceuticals and has undertaken paid lectures for Norgine, Alfa Sigma and Falk Pharma. D.L.S’s institution King’s College London has received grant support from Norgine. J.S.B. has been an advisor to Valeant, Norgine, Kaleido and Takeda and his institution has received support from Bausch Health, Kaleido, Grifols and Mallinckrodt pharmaceuticals. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

