An mRNA-based vaccine candidate against SARS-CoV-2 elicits stable immuno-response with single dose
- PMID: 34039497
- PMCID: PMC8130517
- DOI: 10.1016/j.vaccine.2021.05.035
An mRNA-based vaccine candidate against SARS-CoV-2 elicits stable immuno-response with single dose
Abstract
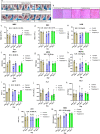
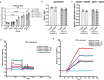
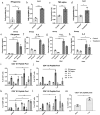
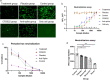
D614G genotype of SARS-CoV-2 virus is highly infectious and responsible for almost all infection for 2nd wave. However, there are currently no reports with D614G as vaccine candidate. Here we report the development of an mRNA-LNP vaccine with D614G variant and characterization in animal model. We have used special mRNA-architecture and formulation that provides suitable response of the product. The surface plasmon resonance (SPR) data with spike protein (S) revealed that immunization generated specific antibody pools against the whole extracellular domain (RBD and S2) of the spike protein. The anti-sera and purified IgGs from immunized mice neutralized SARS-CoV-2-pseudoviruses in ACE2-expressing HEK293 cells in a dose dependent manner. Importantly, single-dose immunization protected mice-lungs from homotypic-pseudovirus entry and cytopathy. The immunologic responses have been implicated by a balanced and stable population of CD4+ cells with a Th1 bias. The data suggested great promise for immediate translation of the technology to the clinic.
Keywords: COVID; Coronavirus; D614G; Immunization; LNP; Lipid nanoparticle; Vaccination.
Copyright © 2021 Globe Biotech Limited. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice.Immunity. 2020 Oct 13;53(4):724-732.e7. doi: 10.1016/j.immuni.2020.07.019. Epub 2020 Jul 30. Immunity. 2020. PMID: 32783919 Free PMC article.
-
The Antigenicity of Epidemic SARS-CoV-2 Variants in the United Kingdom.Front Immunol. 2021 Jun 17;12:687869. doi: 10.3389/fimmu.2021.687869. eCollection 2021. Front Immunol. 2021. PMID: 34220844 Free PMC article.
-
RBD-mRNA vaccine induces broadly neutralizing antibodies against Omicron and multiple other variants and protects mice from SARS-CoV-2 challenge.Transl Res. 2022 Oct;248:11-21. doi: 10.1016/j.trsl.2022.04.007. Epub 2022 Apr 28. Transl Res. 2022. PMID: 35489692 Free PMC article.
-
A self-amplifying mRNA SARS-CoV-2 vaccine candidate induces safe and robust protective immunity in preclinical models.Mol Ther. 2022 May 4;30(5):1897-1912. doi: 10.1016/j.ymthe.2022.01.001. Epub 2022 Jan 3. Mol Ther. 2022. PMID: 34990810 Free PMC article. Clinical Trial.
-
A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity.Nature. 2020 Oct;586(7830):572-577. doi: 10.1038/s41586-020-2599-8. Epub 2020 Jul 29. Nature. 2020. PMID: 32726802
Cited by
-
COVID-19 mRNA vaccines: Platforms and current developments.Mol Ther. 2022 May 4;30(5):1850-1868. doi: 10.1016/j.ymthe.2022.02.016. Epub 2022 Feb 19. Mol Ther. 2022. PMID: 35189345 Free PMC article. Review.
-
Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity.Vaccines (Basel). 2024 Feb 12;12(2):186. doi: 10.3390/vaccines12020186. Vaccines (Basel). 2024. PMID: 38400169 Free PMC article. Review.
-
Dental Healthcare Amid the COVID-19 Pandemic.Int J Environ Res Public Health. 2021 Oct 20;18(21):11008. doi: 10.3390/ijerph182111008. Int J Environ Res Public Health. 2021. PMID: 34769526 Free PMC article. Review.
-
Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies.Biomed Pharmacother. 2022 Feb;146:112507. doi: 10.1016/j.biopha.2021.112507. Epub 2021 Dec 7. Biomed Pharmacother. 2022. PMID: 34891122 Free PMC article. Review.
-
DoE-derived continuous and robust process for manufacturing of pharmaceutical-grade wide-range LNPs for RNA-vaccine/drug delivery.Sci Rep. 2022 Jun 7;12(1):9394. doi: 10.1038/s41598-022-12100-z. Sci Rep. 2022. PMID: 35672337 Free PMC article.
References
-
- “COVID-19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum,” Feb. 2020. Accessed: Sep. 09, 2020. [Online]. Available: https://www.who.int/publications/m/item/covid-19-public-health-emergency....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

