Protein tyrosine phosphatase receptor type C (PTPRC or CD45)
- PMID: 34039664
- PMCID: PMC8380896
- DOI: 10.1136/jclinpath-2020-206927
Protein tyrosine phosphatase receptor type C (PTPRC or CD45)
Abstract
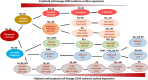
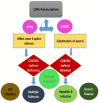
The leucocyte common antigen, protein tyrosine phosphatase receptor type C (PTPRC), also known as CD45, is a transmembrane glycoprotein, expressed on almost all haematopoietic cells except for mature erythrocytes, and is an essential regulator of T and B cell antigen receptor-mediated activation. Disruption of the equilibrium between protein tyrosine kinase and phosphatase activity (from CD45 and others) can result in immunodeficiency, autoimmunity, or malignancy. CD45 is normally present on the cell surface, therefore it works upstream of a large signalling network which differs between cell types, and thus the effects of CD45 on these cells are also different. However, it is becoming clear that CD45 plays an essential role in the innate immune system and this is likely to be a key area for future research. In this review of PTPRC (CD45), its structure and biological activities as well as abnormal expression of CD45 in leukaemia and lymphoma will be discussed.
Keywords: hematology; leukemia; myeloid; myeloproliferative disorders.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Hall LR, Streuli M, Schlossman SF, et al. . Complete exon-intron organization of the human leukocyte common antigen (CD45) gene. J Immunol 1988;141:2781–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
