Oviductal motile cilia are essential for oocyte pickup but dispensable for sperm and embryo transport
- PMID: 34039711
- PMCID: PMC8179221
- DOI: 10.1073/pnas.2102940118
Oviductal motile cilia are essential for oocyte pickup but dispensable for sperm and embryo transport
Abstract
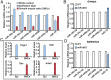
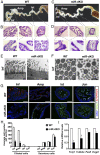
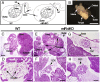
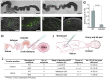
Mammalian oviducts play an essential role in female fertility by picking up ovulated oocytes and transporting and nurturing gametes (sperm/oocytes) and early embryos. However, the relative contributions to these functions from various cell types within the oviduct remain controversial. The oviduct in mice deficient in two microRNA (miRNA) clusters (miR-34b/c and miR-449) lacks cilia, thus allowing us to define the physiological role of oviductal motile cilia. Here, we report that the infundibulum without functional motile cilia failed to pick up the ovulated oocytes. In the absence of functional motile cilia, sperm could still reach the ampulla region, and early embryos managed to migrate to the uterus, but the efficiency was reduced. Further transcriptomic analyses revealed that the five messenger ribonucleic acids (mRNAs) encoded by miR-34b/c and miR-449 function to stabilize a large number of mRNAs involved in cilium organization and assembly and that Tubb4b was one of their target genes. Our data demonstrate that motile cilia in the infundibulum are essential for oocyte pickup and thus, female fertility, whereas motile cilia in other parts of the oviduct facilitate gamete and embryo transport but are not absolutely required for female fertility.
Keywords: Fallopian tube; ciliopathy; fertility; microRNAs; multiciliogenesis.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Stewart C. A., Behringer R. R., “Mouse oviduct development” in Mouse Development, Kubiak J. Z., Ed. (Springer-Verlag, Berlin, Heidelberg, 2012), chap. 14, pp. 247–262. - PubMed
-
- Halbert S. A., Becker D. R., Szal S. E., Ovum transport in the rat oviductal ampulla in the absence of muscle contractility. Biol. Reprod. 40, 1131–1136 (1989). - PubMed
-
- Halbert S. A., Tam P. Y., Blandau R. J., Egg transport in the rabbit oviduct: The roles of cilia and muscle. Science 191, 1052–1053 (1976). - PubMed
-
- Baker K., Beales P. L., Making sense of cilia in disease: The human ciliopathies. Am. J. Med. Genet. C. Semin. Med. Genet. 151C, 281–295 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

