Management of belantamab mafodotin-associated corneal events in patients with relapsed or refractory multiple myeloma (RRMM)
- PMID: 34039952
- PMCID: PMC8155129
- DOI: 10.1038/s41408-021-00494-4
Management of belantamab mafodotin-associated corneal events in patients with relapsed or refractory multiple myeloma (RRMM)
Abstract
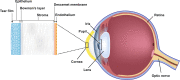
Belantamab mafodotin (belamaf) demonstrated deep and durable responses in patients with heavily pretreated relapsed or refractory multiple myeloma (RRMM) in DREAMM-2 (NCT03525678). Corneal events, specifically keratopathy (including superficial punctate keratopathy and/or microcyst-like epithelial changes (MECs), eye examination findings with/without symptoms), were common, consistent with reports from other antibody-drug conjugates. Given the novel nature of corneal events in RRMM management, guidelines are required for their prompt identification and appropriate management. Eye examination findings from DREAMM-2 and insights from hematology/oncology investigators and ophthalmologists, including corneal specialists, were collated and used to develop corneal event management guidelines. The following recommendations were formulated: close collaboration among hematologist/oncologists and eye care professionals is needed, in part, to provide optimal care in relation to the belamaf benefit-risk profile. Patients receiving belamaf should undergo eye examinations before and during every treatment cycle and promptly upon worsening of symptoms. Severity of corneal events should be determined based on corneal examination findings and changes in best-corrected visual acuity. Treatment decisions, including dose modifications, should be based on the most severe finding present. These guidelines are recommended for the assessment and management of belamaf-associated ocular events to help mitigate ocular risk and enable patients to continue to experience a clinical benefit with belamaf.
Conflict of interest statement
S.L has received grant funding and personal fees from Celgene and Takeda, and personal fees from Novartis, Bristol-Myers Squibb, GSK, Amgen, Merck, and Janssen; A.K.N. has received grant funding and personal fees from GSK, Janssen, Bristol-Myers Squibb, Celgene, Takeda, and Amgen; and personal fees from Oncopeptides and Spectrum; B.H.J. has received compensation from GSK as a consultant; H.A.P. has received funding from the National Eye Institute and the Research to Prevent Blindness organization; D.S. has received personal fees from Celgene, Janssen, and Amgen; B.E.Z. consulted for and received honoraria from GSK; R.P. has received grant funding, personal fees, and non-financial support from Takeda; personal fees and non-financial support from Janssen, Celgene, and GSK, and personal fees from AbbVie; and support from the National Institute for Health Research University College London Hospitals Biomedical Research Centre; S.D.E. has received personal fees from GSK; J.By. is an employee of and hold stocks and shares in GSK and hold stocks and share in Adaptimmune and Novartis; J.O., J.Ba., and T.P. are employees of and hold stocks and shares in GSK; I.G. is an employee of and holds stocks and shares in GSK and holds stocks and shares in Novartis; R.D. is a consultant for GSK, Dompé, Novartis, Alcon, and Kala; has ownership in Aramis Biosciences, Claris Biotherapeutics, and GelMEDIX; and has received funding from the NIH, DOD, and Allergan; A.V.F. is a consultant for GSK; K.C. is a consultant for GSK; A.J. consulted for and received honoraria from AbbVie, Adaptive Biotechnologies, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Juno, and Karyopharm; P.T., A.Z.B., and N.S.C. declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

