Simplification of culture conditions and feeder-free expansion of bovine embryonic stem cells
- PMID: 34040070
- PMCID: PMC8155104
- DOI: 10.1038/s41598-021-90422-0
Simplification of culture conditions and feeder-free expansion of bovine embryonic stem cells
Abstract
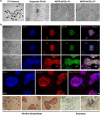
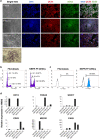
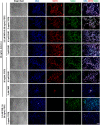
Bovine embryonic stem cells (bESCs) extend the lifespan of the transient pluripotent bovine inner cell mass in vitro. After years of research, derivation of stable bESCs was only recently reported. Although successful, bESC culture relies on complex culture conditions that require a custom-made base medium and mouse embryonic fibroblasts (MEF) feeders, limiting the widespread use of bESCs. We report here simplified bESC culture conditions based on replacing custom base medium with a commercially available alternative and eliminating the need for MEF feeders by using a chemically-defined substrate. bESC lines were cultured and derived using a base medium consisting of N2B27 supplements and 1% BSA (NBFR-bESCs). Newly derived bESC lines were easy to establish, simple to propagate and stable after long-term culture. These cells expressed pluripotency markers and actively proliferated for more than 35 passages while maintaining normal karyotype and the ability to differentiate into derivatives of all three germ lineages in embryoid bodies and teratomas. In addition, NBFR-bESCs grew for multiple passages in a feeder-free culture system based on vitronectin and Activin A medium supplementation while maintaining pluripotency. Simplified conditions will facilitate the use of bESCs for gene editing applications and pluripotency and lineage commitment studies.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Establishment of bovine embryonic stem cells after knockdown of CDX2.Sci Rep. 2016 Jun 20;6:28343. doi: 10.1038/srep28343. Sci Rep. 2016. PMID: 27320776 Free PMC article.
-
Primed bovine embryonic stem cell lines can be derived at diverse stages of blastocyst development with similar efficiency and molecular characteristics.Biol Open. 2025 Mar 15;14(3):BIO061819. doi: 10.1242/bio.061819. Epub 2025 Mar 7. Biol Open. 2025. PMID: 39957479 Free PMC article.
-
Culture conditions and enzymatic passaging of bovine ESC-like cells.Cell Reprogram. 2010 Apr;12(2):151-60. doi: 10.1089/cell.2009.0049. Cell Reprogram. 2010. PMID: 20677930
-
Strategy to Establish Embryo-Derived Pluripotent Stem Cells in Cattle.Int J Mol Sci. 2021 May 9;22(9):5011. doi: 10.3390/ijms22095011. Int J Mol Sci. 2021. PMID: 34065074 Free PMC article. Review.
-
Embryonic stem cells: isolation, characterization and culture.Adv Biochem Eng Biotechnol. 2009;114:173-84. doi: 10.1007/10_2008_20. Adv Biochem Eng Biotechnol. 2009. PMID: 19495683 Review.
Cited by
-
The progress of induced pluripotent stem cells derived from pigs: a mini review of recent advances.Front Cell Dev Biol. 2024 Jun 24;12:1371240. doi: 10.3389/fcell.2024.1371240. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38979033 Free PMC article. Review.
-
In vitro gametogenesis from embryonic stem cells in livestock species: recent advances, opportunities, and challenges to overcome.J Anim Sci. 2023 Jan 3;101:skad137. doi: 10.1093/jas/skad137. J Anim Sci. 2023. PMID: 37140043 Free PMC article. Review.
-
Acquisition and maintenance of pluripotency are influenced by fibroblast growth factor, leukemia inhibitory factor, and 2i in bovine-induced pluripotent stem cells.Front Cell Dev Biol. 2022 Sep 14;10:938709. doi: 10.3389/fcell.2022.938709. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36187479 Free PMC article.
-
Perspectives on scaling production of adipose tissue for food applications.Biomaterials. 2022 Jan;280:121273. doi: 10.1016/j.biomaterials.2021.121273. Epub 2021 Nov 29. Biomaterials. 2022. PMID: 34933254 Free PMC article.
-
Efficient derivation of embryonic stem cells and primordial germ cell-like cells in cattle.J Reprod Dev. 2024 Apr 4;70(2):82-95. doi: 10.1262/jrd.2023-087. Epub 2024 Feb 15. J Reprod Dev. 2024. PMID: 38355134 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

