HDAC6 inhibitors sensitize non-mesenchymal triple-negative breast cancer cells to cysteine deprivation
- PMID: 34040090
- PMCID: PMC8155140
- DOI: 10.1038/s41598-021-90527-6
HDAC6 inhibitors sensitize non-mesenchymal triple-negative breast cancer cells to cysteine deprivation
Abstract
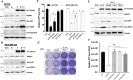
Triple-negative breast cancer (TNBC) is a highly malignant type of breast cancer and lacks effective therapy. Targeting cysteine-dependence is an emerging strategy to treat the mesenchymal TNBC. However, many TNBC cells are non-mesenchymal and unresponsive to cysteine deprivation. To overcome such resistance, three selective HDAC6 inhibitors (Tubacin, CAY10603, and Tubastatin A), identified by epigenetic compound library screening, can synergize with cysteine deprivation to induce cell death in the non-mesenchymal TNBC. Despite the efficacy of HDAC6 inhibitor, knockout of HDAC6 did not mimic the synthetic lethality induced by its inhibitors, indicating that HDAC6 is not the actual target of HDAC6 inhibitor in this context. Instead, transcriptomic profiling showed that tubacin triggers an extensive gene transcriptional program in combination with erastin, a cysteine transport blocker. Notably, the zinc-related gene response along with an increase of labile zinc was induced in cells by the combination treatment. The disturbance of zinc homeostasis was driven by PKCγ activation, which revealed that the PKCγ signaling pathway is required for HDAC6 inhibitor-mediated synthetic lethality. Overall, our study identifies a novel function of HDAC6 inhibitors that function as potent sensitizers of cysteine deprivation and are capable of abolishing cysteine-independence in non-mesenchymal TNBC.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

