Enhanced heroin self-administration and distinct dopamine adaptations in female rats
- PMID: 34040157
- PMCID: PMC8358024
- DOI: 10.1038/s41386-021-01035-0
Enhanced heroin self-administration and distinct dopamine adaptations in female rats
Abstract
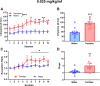
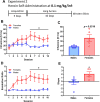
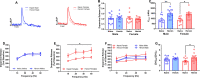
Increasing evidence suggests that females are more vulnerable to the harmful effects of drugs of abuse, including opioids. Additionally, rates of heroin-related deaths substantially increased in females from 1999 to 2017 [1], underscoring the need to evaluate sex differences in heroin vulnerability. Moreover, the neurobiological substrates underlying sexually dimorphic responding to heroin are not fully defined. Thus, we evaluated male and female Long Evans rats on acquisition, dose-responsiveness, and seeking for heroin self-administration (SA) as well as using a long access model to assess escalation of intake at low and high doses of heroin, 0.025 and 0.1 mg/kg/inf, respectively. We paired this with ex vivo fast-scan cyclic voltammetry (FSCV) in the medial nucleus accumbens (NAc) shell and quantification of mu-opioid receptor (MOR) protein in the ventral tegmental area (VTA) and NAc. While males and females had similar heroin SA acquisition rates, females displayed increased responding and intake across doses, seeking for heroin, and escalation on long access. However, we found that males and females had similar expression levels of MORs in the VTA and NAc, regardless of heroin exposure. FSCV results revealed that heroin exposure did not change single-pulse elicited dopamine release, but caused an increase in dopamine transporter activity in both males and females compared to their naïve counterparts. Phasic-like stimulations elicited robust increases in dopamine release in heroin-exposed females compared to heroin-naïve females, with no differences seen in males. Together, our results suggest that differential adaptations of dopamine terminals may underlie the increased heroin SA behaviors seen in females.
© 2021. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Figures


Comment in
-
Adding dopamine to the complexity of sex differences in opioid reinforcement.Neuropsychopharmacology. 2021 Sep;46(10):1705-1706. doi: 10.1038/s41386-021-01060-z. Epub 2021 Jun 18. Neuropsychopharmacology. 2021. PMID: 34145403 Free PMC article. No abstract available.
Similar articles
-
Altered Accumbal Dopamine Terminal Dynamics Following Chronic Heroin Self-Administration.Int J Mol Sci. 2022 Jul 23;23(15):8106. doi: 10.3390/ijms23158106. Int J Mol Sci. 2022. PMID: 35897682 Free PMC article.
-
Augmentation of Heroin Seeking Following Chronic Food Restriction in the Rat: Differential Role for Dopamine Transmission in the Nucleus Accumbens Shell and Core.Neuropsychopharmacology. 2017 Apr;42(5):1136-1145. doi: 10.1038/npp.2016.250. Epub 2016 Nov 8. Neuropsychopharmacology. 2017. PMID: 27824052 Free PMC article.
-
Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2.Eur J Neurosci. 2007 Apr;25(7):2191-200. doi: 10.1111/j.1460-9568.2007.05470.x. Eur J Neurosci. 2007. PMID: 17419755
-
The effects of chronic buprenorphine on intake of heroin and cocaine in rats and its effects on nucleus accumbens dopamine levels during self-administration.Psychopharmacology (Berl). 2006 Sep;188(1):28-41. doi: 10.1007/s00213-006-0485-1. Epub 2006 Aug 11. Psychopharmacology (Berl). 2006. PMID: 16902770
-
Micro-opioid receptor alkylation in the ventral pallidum and ventral tegmental area, but not in the nucleus accumbens, attenuates the effects of heroin on cocaine self-administration in rats.Neuropsychopharmacology. 2008 Apr;33(5):1171-8. doi: 10.1038/sj.npp.1301490. Epub 2007 Jun 20. Neuropsychopharmacology. 2008. PMID: 17581528 Free PMC article.
Cited by
-
Chronic inflammatory pain promotes place preference for fentanyl in male rats but does not change fentanyl self-administration in male and female rats.Neuropharmacology. 2023 Jun 15;231:109512. doi: 10.1016/j.neuropharm.2023.109512. Epub 2023 Mar 21. Neuropharmacology. 2023. PMID: 36948356 Free PMC article.
-
Disruption of the CRF1 receptor eliminates morphine-induced sociability deficits and firing of oxytocinergic neurons in male mice.Elife. 2025 Feb 5;13:RP100849. doi: 10.7554/eLife.100849. Elife. 2025. PMID: 39907358 Free PMC article.
-
Fentanyl self-administration is accelerated by methamphetamine co-use and results in worsened hypodopaminergia in male, but not female rats.Eur J Neurosci. 2024 Oct;60(8):5912-5926. doi: 10.1111/ejn.16533. Epub 2024 Sep 9. Eur J Neurosci. 2024. PMID: 39251212
-
Altered Accumbal Dopamine Terminal Dynamics Following Chronic Heroin Self-Administration.Int J Mol Sci. 2022 Jul 23;23(15):8106. doi: 10.3390/ijms23158106. Int J Mol Sci. 2022. PMID: 35897682 Free PMC article.
-
Sex/Gender Differences in the Time-Course for the Development of Substance Use Disorder: A Focus on the Telescoping Effect.Pharmacol Rev. 2023 Mar;75(2):217-249. doi: 10.1124/pharmrev.121.000361. Epub 2022 Dec 12. Pharmacol Rev. 2023. PMID: 36781217 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

