Metabolic-endocrine disruption due to preterm birth impacts growth, body composition, and neonatal outcome
- PMID: 34040160
- PMCID: PMC9197767
- DOI: 10.1038/s41390-021-01566-8
Metabolic-endocrine disruption due to preterm birth impacts growth, body composition, and neonatal outcome
Abstract
Despite optimized nutrition, preterm-born infants grow slowly and tend to over-accrete body fat. We hypothesize that the premature dissociation of the maternal-placental-fetal unit disrupts the maintenance of physiological endocrine function in the fetus, which has severe consequences for postnatal development. This review highlights the endocrine interactions of the maternal-placental-fetal unit and the early perinatal period in both preterm and term infants. We report on hormonal levels (including tissue, thyroid, adrenal, pancreatic, pituitary, and placental hormones) and nutritional supply and their impact on infant body composition. The data suggest that the premature dissociation of the maternal-placental-fetal unit leads to a clinical picture similar to panhypopituitarism. Further, we describe how the premature withdrawal of the maternal-placental unit, neonatal morbidities, and perinatal stress can cause differences in the levels of growth-promoting hormones, particularly insulin-like growth factors (IGF). In combination with the endocrine disruption that occurs following dissociation of the maternal-placental-fetal unit, the premature adaptation to the extrauterine environment leads to early and fast accretion of fat mass in an immature body. In addition, we report on interventional studies that have aimed to compensate for hormonal deficiencies in infants born preterm through IGF therapy, resulting in improved neonatal morbidity and growth. IMPACT: Preterm birth prematurely dissociates the maternal-placental-fetal unit and disrupts the metabolic-endocrine maintenance of the immature fetus with serious consequences for growth, body composition, and neonatal outcomes. The preterm metabolic-endocrine disruption induces symptoms resembling anterior pituitary failure (panhypopituitarism) with low levels of IGF-1, excessive postnatal fat mass accretion, poor longitudinal growth, and failure to thrive. Appropriate gestational age-adapted nutrition alone seems insufficient for the achievement of optimal growth of preterm infants. Preliminary results from interventional studies show promising effects of early IGF-1 supplementation on postnatal development and neonatal outcomes.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

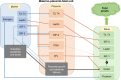
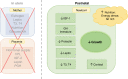
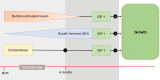
Similar articles
-
Influence of birth-related maternal and neonatal factors on the levels of energy metabolism mediators in infants born at 32 or fewer weeks of gestation.J Matern Fetal Neonatal Med. 2023 Dec;36(2):2290919. doi: 10.1080/14767058.2023.2290919. Epub 2023 Dec 10. J Matern Fetal Neonatal Med. 2023. PMID: 38073078
-
Inflammation induces stunting by lowering bone mass via GH/IGF-1 inhibition in very preterm infants.Pediatr Res. 2023 Sep;94(3):1136-1144. doi: 10.1038/s41390-023-02559-5. Epub 2023 Mar 20. Pediatr Res. 2023. PMID: 36941338
-
Association of maternal body composition and diet on breast milk hormones and neonatal growth during the first month of lactation.Front Endocrinol (Lausanne). 2023 Mar 2;14:1090499. doi: 10.3389/fendo.2023.1090499. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36936154 Free PMC article.
-
Do preterm girls need different nutrition to preterm boys? Sex-specific nutrition for the preterm infant.Pediatr Res. 2021 Jan;89(2):313-317. doi: 10.1038/s41390-020-01252-1. Epub 2020 Nov 12. Pediatr Res. 2021. PMID: 33184497 Review.
-
Late preterm: a new high risk group in neonatology.J Matern Fetal Neonatal Med. 2021 Aug;34(16):2717-2730. doi: 10.1080/14767058.2019.1670796. Epub 2019 Oct 1. J Matern Fetal Neonatal Med. 2021. PMID: 31575303 Review.
Cited by
-
Pituitary gland height evaluated with magnetic resonance imaging in premature twins: the impact of growth and sex.Pediatr Radiol. 2024 May;54(5):787-794. doi: 10.1007/s00247-024-05873-0. Epub 2024 Feb 22. Pediatr Radiol. 2024. PMID: 38386022
-
Evaluation of Placental Toxicity of Five Essential Oils and Their Potential Endocrine-Disrupting Effects.Curr Issues Mol Biol. 2022 Jun 28;44(7):2794-2810. doi: 10.3390/cimb44070192. Curr Issues Mol Biol. 2022. PMID: 35877416 Free PMC article.
-
Glucose-regulatory hormones and growth in very preterm infants fed fortified human milk.Pediatr Res. 2024 Aug;96(3):713-722. doi: 10.1038/s41390-024-03166-8. Epub 2024 Apr 5. Pediatr Res. 2024. PMID: 38580842 Free PMC article. Clinical Trial.
-
Expected and Desirable Preterm and Small Infant Growth Patterns.Adv Nutr. 2024 Jun;15(6):100220. doi: 10.1016/j.advnut.2024.100220. Epub 2024 Apr 24. Adv Nutr. 2024. PMID: 38670164 Free PMC article. Review.
-
Impact of Maternal Ultra-Processed Food Consumption and Preterm Birth on the Development of Metabolic Disorders in Offspring.J Pediatr Perinatol Child Health. 2025;9(2):68-84. doi: 10.26502/jppch.74050214. Epub 2025 Apr 28. J Pediatr Perinatol Child Health. 2025. PMID: 40519768 Free PMC article.
References
-
- American Academy of Pediatrics. Committee on Nutrition. Pediatric Nutrition 7th edn (American Academy of Pediatrics, 2013).
-
- Algotar A, et al. Unique patterns of body composition and anthropometric measurements during maturation in neonatal intensive care unit neonates: opportunities for modifying nutritional therapy and influencing clinical outcomes. J. Parenter. Enter. Nutr. 2018;42:231–238. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

