MARK4 controls ischaemic heart failure through microtubule detyrosination
- PMID: 34040253
- PMCID: PMC7612144
- DOI: 10.1038/s41586-021-03573-5
MARK4 controls ischaemic heart failure through microtubule detyrosination
Abstract
Myocardial infarction is a major cause of premature death in adults. Compromised cardiac function after myocardial infarction leads to chronic heart failure with systemic health complications and a high mortality rate1. Effective therapeutic strategies are needed to improve the recovery of cardiac function after myocardial infarction. More specifically, there is a major unmet need for a new class of drugs that can improve cardiomyocyte contractility, because inotropic therapies that are currently available have been associated with high morbidity and mortality in patients with systolic heart failure2,3 or have shown a very modest reduction of risk of heart failure4. Microtubule detyrosination is emerging as an important mechanism for the regulation of cardiomyocyte contractility5. Here we show that deficiency of microtubule-affinity regulating kinase 4 (MARK4) substantially limits the reduction in the left ventricular ejection fraction after acute myocardial infarction in mice, without affecting infarct size or cardiac remodelling. Mechanistically, we provide evidence that MARK4 regulates cardiomyocyte contractility by promoting phosphorylation of microtubule-associated protein 4 (MAP4), which facilitates the access of vasohibin 2 (VASH2)-a tubulin carboxypeptidase-to microtubules for the detyrosination of α-tubulin. Our results show how the detyrosination of microtubules in cardiomyocytes is finely tuned by MARK4 to regulate cardiac inotropy, and identify MARK4 as a promising therapeutic target for improving cardiac function after myocardial infarction.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

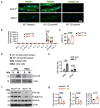
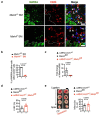

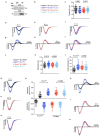

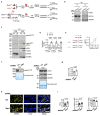







References
-
- Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. - PubMed
-
- Packer M, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325:1468–1475. - PubMed
-
- Cohn JN, et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N Engl J Med. 1998;339:1810–1816. - PubMed
-
- Teerlink JR, et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N Engl J Med. 2021;384:105–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

