Ginkgo biloba Extract 50 (GBE50) Ameliorates Insulin Resistance, Hepatic Steatosis and Liver Injury in High Fat Diet-Fed Mice
- PMID: 34040411
- PMCID: PMC8139725
- DOI: 10.2147/JIR.S302934
Ginkgo biloba Extract 50 (GBE50) Ameliorates Insulin Resistance, Hepatic Steatosis and Liver Injury in High Fat Diet-Fed Mice
Abstract
Background: Ginkgo biloba extract 50 (GBE50) has a variety of pharmacological functions such as anti-inflammatory, antioxidant and maintenance of glucose and lipid metabolism homeostasis. However, the therapeutic effects and mechanisms of GBE50 on non-alcoholic fatty liver disease (NAFLD) remain unknown. Therefore, in this study, we evaluated the therapeutic effects of GBE50 in NAFLD by using a high-fat diet (HFD) mice model.
Methods: C57BL/6J mice were fed a HFD diet for 15 weeks and were given respectively 25, 50, and 100 mg/kg GBE50 daily by gavage from 3 to 15 weeks. After the administration, blood samples and liver tissues were collected for biochemical detection, histological measurement, immunohistochemistry and Western blot, respectively.
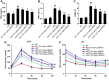
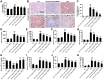
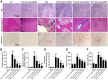
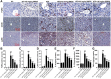
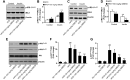
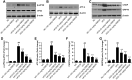
Results: We found that GBE50 treatment could ameliorate insulin resistance (IR), glucose intolerance, lipid accumulation, hepatic steatosis and liver injury in HFD-fed mice. Further mechanism exploration discovered that the hepatoprotective effects of GBE50 on NAFLD may be related to the strengthening of IRS-1 signal activation and the weakening of NF-κB, Akt and endoplasmic reticulum stress signals activation.
Conclusion: GBE50 is a potentially powerful therapeutic agent for the treatment of NAFLD.
Keywords: Ginkgo biloba extract 50; hepatic steatosis; inflammatory response; insulin resistance; liver injury; non-alcoholic fatty liver disease.
© 2021 Li et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Ginkgo biloba Extract GBE50 ameliorates cerebrovascular dysfunction and cognitive impairment in a mouse model of Alzheimer's disease.Phytomedicine. 2025 Jun;141:156646. doi: 10.1016/j.phymed.2025.156646. Epub 2025 Mar 19. Phytomedicine. 2025. PMID: 40138777
-
Protective effects of Ginkgo biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms.Int J Biol Macromol. 2015 Sep;80:573-80. doi: 10.1016/j.ijbiomac.2015.05.054. Epub 2015 Jun 3. Int J Biol Macromol. 2015. PMID: 26047899
-
Effects of Chinese Medicinal Formula BNG-1 on Phosphodiesterase 3B Expression, Hepatic Steatosis, and Insulin Resistance in High Fat Diet-induced NAFLD Mice.Int J Med Sci. 2018 Jul 30;15(11):1194-1202. doi: 10.7150/ijms.26941. eCollection 2018. Int J Med Sci. 2018. PMID: 30123057 Free PMC article.
-
Targeting DUSP7 signaling alleviates hepatic steatosis, inflammation and oxidative stress in high fat diet (HFD)-fed mice via suppression of TAK1.Free Radic Biol Med. 2020 Jun;153:140-158. doi: 10.1016/j.freeradbiomed.2020.04.009. Epub 2020 Apr 18. Free Radic Biol Med. 2020. PMID: 32311490
-
LPSF/GQ-02 inhibits the development of hepatic steatosis and inflammation in a mouse model of non-alcoholic fatty liver disease (NAFLD).PLoS One. 2015 Apr 14;10(4):e0123787. doi: 10.1371/journal.pone.0123787. eCollection 2015. PLoS One. 2015. PMID: 25875942 Free PMC article.
Cited by
-
Tianhuang formula reduces the oxidative stress response of NAFLD by regulating the gut microbiome in mice.Front Microbiol. 2022 Sep 21;13:984019. doi: 10.3389/fmicb.2022.984019. eCollection 2022. Front Microbiol. 2022. PMID: 36212891 Free PMC article.
-
Fumigaclavine C ameliorates liver steatosis by attenuating hepatic de novo lipogenesis via modulation of the RhoA/ROCK signaling pathway.BMC Complement Med Ther. 2023 Aug 16;23(1):288. doi: 10.1186/s12906-023-04110-9. BMC Complement Med Ther. 2023. PMID: 37587459 Free PMC article.
-
Ginkgo biloba Extract 50 (GBE50) Exerts Antifibrotic and Antioxidant Effects on Pulmonary Fibrosis in Mice by Regulating Nrf2 and TGF-β1/Smad Pathways.Appl Biochem Biotechnol. 2024 Aug;196(8):4807-4822. doi: 10.1007/s12010-023-04755-9. Epub 2023 Nov 16. Appl Biochem Biotechnol. 2024. PMID: 37971580
-
A comprehensive review on phytochemicals for fatty liver: are they potential adjuvants?J Mol Med (Berl). 2022 Mar;100(3):411-425. doi: 10.1007/s00109-021-02170-3. Epub 2022 Jan 7. J Mol Med (Berl). 2022. PMID: 34993581 Review.
-
A Cardiac Protection of Germinated Brown Rice During Cardiopulmonary Bypass Surgery and Simulated Myocardial Ischemia.J Inflamm Res. 2021 Jul 15;14:3307-3319. doi: 10.2147/JIR.S321241. eCollection 2021. J Inflamm Res. 2021. PMID: 34290516 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

