Saliva Neurofilament Light Chain Is Not a Diagnostic Biomarker for Neurodegeneration in a Mixed Memory Clinic Population
- PMID: 34040512
- PMCID: PMC8141589
- DOI: 10.3389/fnagi.2021.659898
Saliva Neurofilament Light Chain Is Not a Diagnostic Biomarker for Neurodegeneration in a Mixed Memory Clinic Population
Abstract
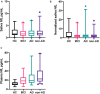
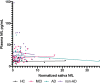
Neurodegeneration and axonal injury result in an increasing release of neurofilament light chain (NfL) into bodily fluids, including cerebrospinal fluid (CSF) and blood. Numerous studies have shown that NfL levels in CSF and blood are increased in neurodegenerative disorders and monitor neurodegeneration. Saliva is an easily accessible biofluid that could be utilized as a biofluid measurement of Alzheimer's disease (AD) biomarkers. In this study, for the first time, salivary NfL was measured and compared to plasma NfL in a consecutive cohort of patients referred to cognitive assessments. In two mixed memory clinic cohorts, saliva samples were taken from 152 patients, AD (n = 49), mild cognitive impairment (MCI) (n = 47), non-AD (n = 56), and also 17 healthy controls. In addition, 135 also had a matching plasma sample. All saliva and plasma samples were analyzed for NfL, and the association between saliva and plasma NfL and CSF levels of total tau (t-tau), phosphorylated tau (p-tau), and beta amyloid 1-42 (Aβ42) were investigated. In total, 162/169 had quantifiable levels of salivary NfL by single molecule array (Simoa). No statistically significant differences were found in salivary NfL concentration across the diagnostic groups, but as expected, significant increases were found for plasma NfL in dementia cases (P < 0.0001). There was no association between saliva and plasma NfL levels. Furthermore, saliva NfL did not correlate with CSF Aβ42, p-tau, or tau concentrations. In conclusion, NfL is detectable in saliva but does not reflect neurodegeneration in the brain.
Keywords: Alzheimer’s disease; biomarker; dementia; neurodegeneration; neurofilament light chain; plasma; saliva.
Copyright © 2021 Gleerup, Sanna, Høgh, Simrén, Blennow, Zetterberg, Hasselbalch, Ashton and Simonsen.
Conflict of interest statement
KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ashton N. J., Leuzy A., Lim Y. M., Troakes C., Hortobágyi T., Höglund K., et al. (2019b). Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol. Commun. 7:5. 10.1186/s40478-018-0649-3 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

