Assessment of Anti-Hypertensive Drug Adherence by Serial Aldosterone-To-Renin Ratio Measurement
- PMID: 34040531
- PMCID: PMC8141919
- DOI: 10.3389/fphar.2021.668843
Assessment of Anti-Hypertensive Drug Adherence by Serial Aldosterone-To-Renin Ratio Measurement
Abstract
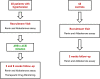
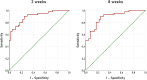
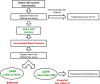
Reduced or absent compliance to anti-hypertensive treatment is a major obstacle to the achievement of blood pressure target in patients with arterial hypertension. Current available methods for therapeutic adherence assessment display low accuracy, limited applicability in clinical practice and/or high costs. We designed a prospective study to evaluate the accuracy of serial measurement of ARR to assess the therapeutic compliance to RAAS inhibitors. We prospectively enrolled 80 subjects: 40 patients with arterial hypertension and 40 normotensive controls. The ARR was evaluated at baseline and 2 and 8 week after initiation of a RAAS inhibitor in patients with hypertension, and at baseline and 2 weeks for the control group. Adherence to the prescribed therapy was confirmed by therapeutic drug monitoring. We observed a significant increase of renin levels and reduction of aldosterone levels after RAAS inhibitors initiation, with consequent reduction of ARR. Delta ARR (ΔARR), defined as relative change in ARR before and after treatment initiation, provided high accuracy for determination of therapeutic compliance, with an AUC of 0.900 at 2 weeks and 0.886 at 8 weeks. A cut-off of -48% of ΔARR provided 90% sensitivity and 75% specificity, at 2 and 8 weeks. In conclusion, the measurement of ΔARR is a powerful test, cheap and widely available to accurately identify the non-adherence to RAAS inhibitors treatment. Herein we propose the implementation of ΔARR in clinical practice through a multi-step flow-chart for the management of patients with uncontrolled blood pressure, with identification of those suspected of non-adherence, reserving therapeutic drug monitoring for non-adherence confirmation.
Keywords: aldosterone; angiotensin receptor blocker; angiotensin-converting enzyme inhibitor; anti-hypertensive treatment; drug adherence; renin; therapeutic drug monitoring.
Copyright © 2021 Buffolo, Sconfienza, Burrello, Losano, Mengozzi, Priolo, Avataneo, D’Avolio, Veglio, Rabbia, Mulatero and Monticone.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
β-Blocker withdrawal is preferable for accurate interpretation of the aldosterone-renin ratio in chronically treated hypertension.Clin Endocrinol (Oxf). 2016 Mar;84(3):325-31. doi: 10.1111/cen.12882. Epub 2015 Sep 22. Clin Endocrinol (Oxf). 2016. PMID: 26300226
-
[Aldosterone-to-renin ratio threshold for screening primary aldosteronism in Chinese hypertensive patients].Zhonghua Xin Xue Guan Bing Za Zhi. 2006 Oct;34(10):868-72. Zhonghua Xin Xue Guan Bing Za Zhi. 2006. PMID: 17217708 Chinese.
-
Test characteristics of the aldosterone-to-renin ratio as a screening test for primary aldosteronism.J Hypertens. 2014 Jan;32(1):115-26. doi: 10.1097/HJH.0b013e3283656b54. J Hypertens. 2014. PMID: 24018605
-
Inhibition of the renin angiotensin aldosterone system: focus on aliskiren.J Assoc Physicians India. 2010 Feb;58:102-8. J Assoc Physicians India. 2010. PMID: 20653151 Review.
-
Arterial stiffness and the renin-angiotensin-aldosterone system.J Renin Angiotensin Aldosterone Syst. 2004 Sep;5(3):102-8. doi: 10.3317/jraas.2004.025. J Renin Angiotensin Aldosterone Syst. 2004. PMID: 15526244 Review.
Cited by
-
Chemical adherence testing in the clinical management of hypertension: a scoping review.Front Pharmacol. 2024 Nov 6;15:1452464. doi: 10.3389/fphar.2024.1452464. eCollection 2024. Front Pharmacol. 2024. PMID: 39568584 Free PMC article.
-
Prescribing characteristics and guideline concordance of antihypertensive western and Chinese patent medicine in Internet hospitals in China: a cross-sectional study.Front Pharmacol. 2025 Apr 28;16:1580787. doi: 10.3389/fphar.2025.1580787. eCollection 2025. Front Pharmacol. 2025. PMID: 40371350 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

