Optimising Follicular Development, Pituitary Suppression, Triggering and Luteal Phase Support During Assisted Reproductive Technology: A Delphi Consensus
- PMID: 34040586
- PMCID: PMC8142593
- DOI: 10.3389/fendo.2021.675670
Optimising Follicular Development, Pituitary Suppression, Triggering and Luteal Phase Support During Assisted Reproductive Technology: A Delphi Consensus
Abstract
Background: A Delphi consensus was conducted to evaluate global expert opinions on key aspects of assisted reproductive technology (ART) treatment.
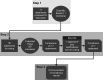
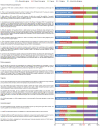
Methods: Ten experts plus the Scientific Coordinator discussed and amended statements plus supporting references proposed by the Scientific Coordinator. The statements were distributed via an online survey to 35 experts, who voted on their level of agreement or disagreement with each statement. Consensus was reached if the proportion of participants agreeing or disagreeing with a statement was >66%.
Results: Eighteen statements were developed. All statements reached consensus and the most relevant are summarised here. (1) Follicular development and stimulation with gonadotropins (n = 9 statements): Recombinant human follicle stimulating hormone (r-hFSH) alone is sufficient for follicular development in normogonadotropic patients aged <35 years. Oocyte number and live birth rate are strongly correlated; there is a positive linear correlation with cumulative live birth rate. Different r-hFSH preparations have identical polypeptide chains but different glycosylation patterns, affecting the biospecific activity of r-hFSH. r-hFSH plus recombinant human LH (r-hFSH:r-hLH) demonstrates improved pregnancy rates and cost efficacy versus human menopausal gonadotropin (hMG) in patients with severe FSH and LH deficiency. (2) Pituitary suppression (n = 2 statements): Gonadotropin releasing hormone (GnRH) antagonists are associated with lower rates of any grade ovarian hyperstimulation syndrome (OHSS) and cycle cancellation versus GnRH agonists. (3) Final oocyte maturation triggering (n=4 statements): Human chorionic gonadotropin (hCG) represents the gold standard in fresh cycles. The efficacy of hCG triggering for frozen transfers in modified natural cycles is controversial compared with LH peak monitoring. Current evidence supports significantly higher pregnancy rates with hCG + GnRH agonist versus hCG alone, but further evidence is needed. GnRH agonist trigger, in GnRH antagonist protocol, is recommended for final oocyte maturation in women at risk of OHSS. (4) Luteal-phase support (n = 3 statements): Vaginal progesterone therapy represents the gold standard for luteal-phase support.
Conclusions: This Delphi consensus provides a real-world clinical perspective on the specific approaches during the key steps of ART treatment from a diverse group of international experts. Additional guidance from clinicians on ART strategies could complement guidelines and policies, and may help to further improve treatment outcomes.
Keywords: assisted reproductive technology (ART); expert opinion; gonadotropins; luteal phase support; oocyte maturation; optimisation; ovarian stimulation; trigger.
Copyright © 2021 Orvieto, Venetis, Fatemi, D’Hooghe, Fischer, Koloda, Horton, Grynberg, Longobardi, Esteves, Sunkara, Li and Alviggi.
Conflict of interest statement
TD and SL are employees of Merck KGaA, Darmstadt, Germany. RO received speaker fees/honoraria from Ferring and Merck KGaA, Darmstadt, Germany. CV is supported by a NHMRC Early Career Fellowship (GNT1147154). He also has equity interests in Virtus Health and reports grants, personal fees and non-financial support from Merck KGaA, Darmstadt, Germany, personal fees and non-financial support from MSD, grants and non-financial support from Ferring, personal fees from Besins Healthcare, and grants and non-financial support from Abbott. HF received speaker fees/honoraria/grants from MSD, Ferring, Besin, Merck KGaA, Darmstadt, Germany, and Sun Pharma. RF received consulting fees and honoraria from Merck KGaA, Darmstadt, Germany. YK received speaker fees/honoraria/grants from MSD, Merck KGaA, Darmstadt, Germany, Besins Healthcare, Gedeon Richter, Abbott, Bayer, and Sun Pharma. MH declares receipt of research grants from Merck KGaA, Darmstadt, Germany, and lecture fees from Merck KGaA, Darmstadt, Germany and Ferring. MG received fees from Merck KGaA, Darmstadt, Germany, Ferring, Gedeon Richter, MSD, IBSA. SE declares receipt of unrestricted research grants from Merck KGaA, Darmstadt, Germany, and lecture fees from Merck KGaA, Darmstadt, Germany, Gedeon Richter and Medical Education Academy. SS was speaker at non-promotional educational symposia by Merck KGaA, Darmstadt, Germany and Ferring in the last 12 months. YL received speaker fees/grants from Merck KGaA, Darmstadt, Germany, MSD, and Bayer. CA received consulting fees and payment/honoraria from Merck KGaA, Darmstadt, Germany.
Figures
Similar articles
-
FSH/LH co-stimulation in Advanced Maternal Age (AMA) and hypo-responder patients - Arabian gulf delphi consensus group.Front Endocrinol (Lausanne). 2024 Dec 12;15:1506332. doi: 10.3389/fendo.2024.1506332. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39726844 Free PMC article.
-
Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment.J Clin Endocrinol Metab. 2003 Sep;88(9):4186-92. doi: 10.1210/jc.2002-021953. J Clin Endocrinol Metab. 2003. PMID: 12970285 Clinical Trial.
-
Endometrial gene expression in the early luteal phase is impacted by mode of triggering final oocyte maturation in recFSH stimulated and GnRH antagonist co-treated IVF cycles.Hum Reprod. 2012 Nov;27(11):3259-72. doi: 10.1093/humrep/des279. Epub 2012 Aug 28. Hum Reprod. 2012. PMID: 22930004 Clinical Trial.
-
The prevention of ovarian hyperstimulation syndrome.J Obstet Gynaecol Can. 2014 Nov;36(11):1024-1033. doi: 10.1016/S1701-2163(15)30417-5. J Obstet Gynaecol Can. 2014. PMID: 25574681 Review. English, French.
-
Gonadotropins.2018 Mar 26. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2018 Mar 26. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31644163 Free Books & Documents. Review.
Cited by
-
Assisted Reproductive Technology and Disease Management in Infertile Women with Multiple Sclerosis.CNS Drugs. 2023 Oct;37(10):849-866. doi: 10.1007/s40263-023-01036-1. Epub 2023 Sep 7. CNS Drugs. 2023. PMID: 37679579 Free PMC article. Review.
-
Progesterone in Pregnancy: Evidence-Based Strategies to Reduce Miscarriage and Enhance Assisted Reproductive Technology.Med Sci Monit. 2024 Mar 8;30:e943400. doi: 10.12659/MSM.943400. Med Sci Monit. 2024. PMID: 38501164 Free PMC article.
-
Interplay Between mTOR and Hippo Signaling in the Ovary: Clinical Choice Guidance Between Different Gonadotropin Preparations for Better IVF.Front Endocrinol (Lausanne). 2021 Jul 21;12:702446. doi: 10.3389/fendo.2021.702446. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34367070 Free PMC article. Review.
-
ACcurate COnsensus Reporting Document (ACCORD) explanation and elaboration: Guidance and examples to support reporting consensus methods.PLoS Med. 2024 May 6;21(5):e1004390. doi: 10.1371/journal.pmed.1004390. eCollection 2024 May. PLoS Med. 2024. PMID: 38709851 Free PMC article.
-
Ovarian Follicular Growth through Intermittent Vaginal Gonadotropin Administration in Diminished Ovarian Reserve Women.Pharmaceutics. 2022 Apr 15;14(4):869. doi: 10.3390/pharmaceutics14040869. Pharmaceutics. 2022. PMID: 35456706 Free PMC article.
References
-
- World Health Organization . World Report on Disability (2011). Available at: https://apps.who.int/iris/bitstream/handle/10665/70670/WHO_NMH_VIP_11.01... (Accessed 27 May 2020).
-
- World Health Organization . Fact Sheet: Fertility (2020). Available at: https://www.who.int/news-room/fact-sheets/detail/infertility (Accessed 27/01/2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

