Digenic Inheritance and Gene-Environment Interaction in a Patient With Hypertriglyceridemia and Acute Pancreatitis
- PMID: 34040631
- PMCID: PMC8143378
- DOI: 10.3389/fgene.2021.640859
Digenic Inheritance and Gene-Environment Interaction in a Patient With Hypertriglyceridemia and Acute Pancreatitis
Abstract
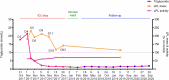
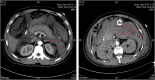
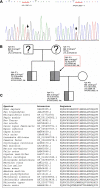
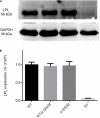
The etiology of hypertriglyceridemia (HTG) and acute pancreatitis (AP) is complex. Herein, we dissected the underlying etiology in a patient with HTG and AP. The patient had a 20-year history of heavy alcohol consumption and an 8-year history of mild HTG. He was hospitalized for alcohol-triggered AP, with a plasma triglyceride (TG) level up to 21.4 mmol/L. A temporary rise in post-heparin LPL concentration (1.5-2.5 times of controls) was noted during the early days of AP whilst LPL activity was consistently low (50∼70% of controls). His TG level rapidly decreased to normal in response to treatment, and remained normal to borderline high during a ∼3-year follow-up period during which he had abstained completely from alcohol. Sequencing of the five primary HTG genes (i.e., LPL, APOC2, APOA5, GPIHBP1 and LMF1) identified two heterozygous variants. One was the common APOA5 c.553G > T (p.Gly185Cys) variant, which has been previously associated with altered TG levels as well as HTG-induced acute pancreatitis (HTG-AP). The other was a rare variant in the LPL gene, c.756T > G (p.Ile252Met), which was predicted to be likely pathogenic and found experimentally to cause a 40% loss of LPL activity without affecting either protein synthesis or secretion. We provide evidence that both a gene-gene interaction (between the common APOA5 variant and the rare LPL variant) and a gene-environment interaction (between alcohol and digenic inheritance) might have contributed to the development of mild HTG and alcohol-triggered AP in the patient, thereby improving our understanding of the complex etiology of HTG and HTG-AP.
Keywords: APOA5 gene; LPL gene; acute pancreatitis; alcohol-induced hypertriglyceridemia; gene-environment interaction.
Copyright © 2021 Yang, Pu, Li, Shi, Chen, Zhang, Hu, Zhou, Chen, Li, Tong, Férec, Cooper, Chen and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The East Asian-specific LPL p.Ala288Thr (c.862G > A) missense variant exerts a mild effect on protein function.Lipids Health Dis. 2023 Aug 7;22(1):119. doi: 10.1186/s12944-023-01875-3. Lipids Health Dis. 2023. PMID: 37550668 Free PMC article.
-
Identification of a novel LPL nonsense variant and further insights into the complex etiology and expression of hypertriglyceridemia-induced acute pancreatitis.Lipids Health Dis. 2020 Apr 7;19(1):63. doi: 10.1186/s12944-020-01249-z. Lipids Health Dis. 2020. PMID: 32264896 Free PMC article.
-
Gene-environment interaction between APOA5 c.553G>T and pregnancy in hypertriglyceridemia-induced acute pancreatitis.J Clin Lipidol. 2020 Jul-Aug;14(4):498-506. doi: 10.1016/j.jacl.2020.05.003. Epub 2020 May 21. J Clin Lipidol. 2020. PMID: 32561169
-
Current knowledge of hypertriglyceridemic pancreatitis.Eur J Intern Med. 2014 Oct;25(8):689-94. doi: 10.1016/j.ejim.2014.08.008. Epub 2014 Sep 27. Eur J Intern Med. 2014. PMID: 25269432 Review.
-
Familial chylomicronemia syndrome: an under-recognized cause of severe hypertriglyceridaemia.J Intern Med. 2020 Apr;287(4):340-348. doi: 10.1111/joim.13016. Epub 2020 Jan 8. J Intern Med. 2020. PMID: 31840878 Review.
Cited by
-
Case Report: Successful Management of a 29-Day-Old Infant With Severe Hyperlipidemia From a Novel Homozygous Variant of GPIHBP1 Gene.Front Pediatr. 2022 Mar 10;10:792574. doi: 10.3389/fped.2022.792574. eCollection 2022. Front Pediatr. 2022. PMID: 35359903 Free PMC article.
-
The East Asian-specific LPL p.Ala288Thr (c.862G > A) missense variant exerts a mild effect on protein function.Lipids Health Dis. 2023 Aug 7;22(1):119. doi: 10.1186/s12944-023-01875-3. Lipids Health Dis. 2023. PMID: 37550668 Free PMC article.
-
Significant but partial lipoprotein lipase functional loss caused by a novel occurrence of rare LPL biallelic variants.Lipids Health Dis. 2024 Apr 1;23(1):92. doi: 10.1186/s12944-024-02086-0. Lipids Health Dis. 2024. PMID: 38561841 Free PMC article.
-
Immune-Enhancing Treatment among Acute Necrotizing Pancreatitis Patients with Metabolic Abnormalities: A Post Hoc Analysis of a Randomized Clinical Trial.Gut Liver. 2024 Sep 15;18(5):906-914. doi: 10.5009/gnl230326. Epub 2024 Feb 15. Gut Liver. 2024. PMID: 38356344 Free PMC article. Clinical Trial.
-
AAV-mediated hepatic LPL expression ameliorates severe hypertriglyceridemia and acute pancreatitis in Gpihbp1 deficient mice and rats.Mol Ther. 2024 Jan 3;32(1):59-73. doi: 10.1016/j.ymthe.2023.11.018. Epub 2023 Nov 15. Mol Ther. 2024. PMID: 37974401 Free PMC article.
References
-
- Beg O. U., Meng M. S., Skarlatos S. I., Previato L., Brunzell J. D., Brewer H. B., Jr., et al. (1990). Lipoprotein lipaseBethesda: a single amino acid substitution (Ala-176>Thr) leads to abnormal heparin binding and loss of enzymic activity. Proc. Natl. Acad. Sci. U.S.A. 87 3474–3478. 10.1073/pnas.87.9.3474 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

