Divergent reactivity of sulfinates with pyridinium salts based on one- versus two-electron pathways
- PMID: 34040737
- PMCID: PMC8132931
- DOI: 10.1039/d1sc00776a
Divergent reactivity of sulfinates with pyridinium salts based on one- versus two-electron pathways
Abstract
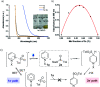
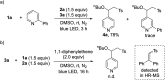
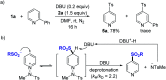
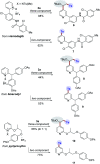
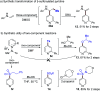
One of the main goals of modern synthesis is to develop distinct reaction pathways from identical starting materials for the efficient synthesis of diverse compounds. Herein, we disclose the unique divergent reactivity of the combination sets of pyridinium salts and sulfinates to achieve sulfonative pyridylation of alkenes and direct C4-sulfonylation of pyridines by controlling the one- versus two-electron reaction manifolds for the selective formation of each product. Base-catalyzed cross-coupling between sulfinates and N-amidopyridinium salts led to the direct introduction of a sulfonyl group into the C4 position of pyridines. Remarkably, the reactivity of this set of compounds is completely altered upon exposure to visible light: electron donor-acceptor complexes of N-amidopyridinium salts and sulfinates are formed to enable access to sulfonyl radicals. In this catalyst-free radical pathway, both sulfonyl and pyridyl groups could be incorporated into alkenes via a three-component reaction, which provides facile access to a variety of β-pyridyl alkyl sulfones. These two reactions are orthogonal and complementary, achieving a broad substrate scope in a late-stage fashion under mild reaction conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
N-Functionalized Pyridinium Salts: A New Chapter for Site-Selective Pyridine C-H Functionalization via Radical-Based Processes under Visible Light Irradiation.Acc Chem Res. 2022 Oct 18;55(20):3043-3056. doi: 10.1021/acs.accounts.2c00530. Epub 2022 Sep 27. Acc Chem Res. 2022. PMID: 36166489 Review.
-
Visible-Light-Driven C4-Selective Alkylation of Pyridinium Derivatives with Alkyl Bromides.J Am Chem Soc. 2020 Jul 1;142(26):11370-11375. doi: 10.1021/jacs.0c04499. Epub 2020 Jun 18. J Am Chem Soc. 2020. PMID: 32530614
-
A visible-light-catalyzed sulfonylation reaction of an aryl selenonium salt via an electron donor-acceptor complex.Org Biomol Chem. 2024 Mar 20;22(12):2451-2455. doi: 10.1039/d4ob00060a. Org Biomol Chem. 2024. PMID: 38419463
-
Sulfinates from Amines: A Radical Approach to Alkyl Sulfonyl Derivatives via Donor-Acceptor Activation of Pyridinium Salts.Org Lett. 2021 Nov 5;23(21):8488-8493. doi: 10.1021/acs.orglett.1c03194. Epub 2021 Oct 14. Org Lett. 2021. PMID: 34648294
-
Synthesis and applications of sodium sulfinates (RSO2Na): a powerful building block for the synthesis of organosulfur compounds.RSC Adv. 2021 Mar 1;11(16):9130-9221. doi: 10.1039/d0ra09759d. eCollection 2021 Mar 1. RSC Adv. 2021. PMID: 35423435 Free PMC article. Review.
Cited by
-
Unified ionic and radical C-4 alkylation and arylation of pyridines.Chem Sci. 2024 Jul 4;15(31):12442-12450. doi: 10.1039/d4sc03739a. eCollection 2024 Aug 7. Chem Sci. 2024. PMID: 39118600 Free PMC article.
-
Nucleophilic C4-selective (hetero) arylation of pyridines for facile synthesis of heterobiaryls.Front Chem. 2023 Sep 1;11:1254632. doi: 10.3389/fchem.2023.1254632. eCollection 2023. Front Chem. 2023. PMID: 37720719 Free PMC article.
-
Enantioselective functionalization at the C4 position of pyridinium salts through NHC catalysis.Nat Commun. 2022 Apr 1;13(1):1776. doi: 10.1038/s41467-022-29462-7. Nat Commun. 2022. PMID: 35365667 Free PMC article.
-
Practical and Regioselective Synthesis of C-4-Alkylated Pyridines.J Am Chem Soc. 2021 Aug 11;143(31):11927-11933. doi: 10.1021/jacs.1c05278. Epub 2021 Jul 28. J Am Chem Soc. 2021. PMID: 34318659 Free PMC article.
-
N-Amino Pyridinium Salts in Organic Synthesis.Org Chem Front. 2023 May 21;10(10):2563-2580. doi: 10.1039/d3qo00190c. Epub 2023 Mar 30. Org Chem Front. 2023. PMID: 37840843 Free PMC article.
References
-
- Liu N.-W. Liang S. Manolikakes G. Synthesis. 2016;48:1939.
-
- Graybill B. M. J. Org. Chem. 1967;32:2931.
- Repichet S. Roux C. L. Hernandez P. Dubac J. J. Org. Chem. 1999;64:6497.
-
- Jereb M. Green Chem. 2012;14:3047.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

