The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells
- PMID: 34041035
- PMCID: PMC8141658
- DOI: 10.3389/fonc.2021.672781
The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells
Abstract
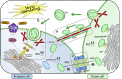
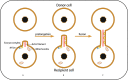
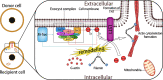
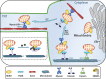
Mitochondria are vital organelles in cells, regulating energy metabolism and apoptosis. Mitochondrial transcellular transfer plays a crucial role during physiological and pathological conditions, such as rescuing recipient cells from bioenergetic deficit and tumorigenesis. Studies have shown several structures that conduct transcellular transfer of mitochondria, including tunneling nanotubes (TNTs), extracellular vesicles (EVs), and Cx43 gap junctions (GJs). The intra- and intercellular transfer of mitochondria is driven by a transport complex. Mitochondrial Rho small GTPase (MIRO) may be the adaptor that connects the transport complex with mitochondria, and myosin XIX is the motor protein of the transport complex, which participates in the transcellular transport of mitochondria through TNTs. In this review, the roles of TNTs, EVs, GJs, and related transport complexes in mitochondrial transcellular transfer are discussed in detail, as well as the formation mechanisms of TNTs and EVs. This review provides the basis for the development of potential clinical therapies targeting the structures of mitochondrial transcellular transfer.
Keywords: Cx43 gap junction; Miro; extracellular vesicles; mitochondria; myosin XIX; transcellular transport; tunneling nanotubes.
Copyright © 2021 Qin, Jiang, Yang, Zhao, Zhou and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Inter and Intracellular mitochondrial trafficking in health and disease.Ageing Res Rev. 2020 Sep;62:101128. doi: 10.1016/j.arr.2020.101128. Epub 2020 Jul 23. Ageing Res Rev. 2020. PMID: 32712108 Free PMC article. Review.
-
Tunneling nanotubes and mesenchymal stem cells: New insights into the role of melatonin in neuronal recovery.J Pineal Res. 2022 Aug;73(1):e12800. doi: 10.1111/jpi.12800. Epub 2022 Apr 22. J Pineal Res. 2022. PMID: 35419879 Free PMC article. Review.
-
Extracellular Vesicles and Cx43-Gap Junction Channels Are the Main Routes for Mitochondrial Transfer from Ultra-Purified Mesenchymal Stem Cells, RECs.Int J Mol Sci. 2023 Jun 18;24(12):10294. doi: 10.3390/ijms241210294. Int J Mol Sci. 2023. PMID: 37373439 Free PMC article.
-
Horizontal mitochondrial transfer as a novel bioenergetic tool for mesenchymal stromal/stem cells: molecular mechanisms and therapeutic potential in a variety of diseases.J Transl Med. 2024 May 24;22(1):491. doi: 10.1186/s12967-024-05047-4. J Transl Med. 2024. PMID: 38790026 Free PMC article. Review.
-
Miro proteins and their role in mitochondrial transfer in cancer and beyond.Front Cell Dev Biol. 2022 Jul 25;10:937753. doi: 10.3389/fcell.2022.937753. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35959487 Free PMC article. Review.
Cited by
-
Mitochondria on the move: Horizontal mitochondrial transfer in disease and health.J Cell Biol. 2023 Mar 6;222(3):e202211044. doi: 10.1083/jcb.202211044. Epub 2023 Feb 16. J Cell Biol. 2023. PMID: 36795453 Free PMC article.
-
Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo.Cell Rep. 2022 Apr 12;39(2):110651. doi: 10.1016/j.celrep.2022.110651. Cell Rep. 2022. PMID: 35417683 Free PMC article.
-
The Potential Use of Mitochondrial Extracellular Vesicles as Biomarkers or Therapeutical Tools.Int J Mol Sci. 2023 Apr 10;24(8):7005. doi: 10.3390/ijms24087005. Int J Mol Sci. 2023. PMID: 37108168 Free PMC article. Review.
-
Platelet-derived microparticles provoke chronic lymphocytic leukemia malignancy through metabolic reprogramming.Front Immunol. 2023 Jun 27;14:1207631. doi: 10.3389/fimmu.2023.1207631. eCollection 2023. Front Immunol. 2023. PMID: 37441073 Free PMC article.
-
Large Fibrous Connective Tissue Reduces Oxidative Stress to Form a Living Cell Scaffold in Adipose Grafts.Antioxidants (Basel). 2025 Feb 26;14(3):270. doi: 10.3390/antiox14030270. Antioxidants (Basel). 2025. PMID: 40227213 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

