Phase I Trial on Arterial Embolization with Hypoxia Activated Tirapazamine for Unresectable Hepatocellular Carcinoma
- PMID: 34041204
- PMCID: PMC8139681
- DOI: 10.2147/JHC.S304275
Phase I Trial on Arterial Embolization with Hypoxia Activated Tirapazamine for Unresectable Hepatocellular Carcinoma
Abstract
Background: Tirapazamine (TPZ) is a hypoxia activated drug that may be synergistic with transarterial embolization (TAE). The primary objective was to evaluate the safety of combining TPZ and TAE in patients with unresectable HCC and determine the optimal dose for Phase II.
Methods: This was a Phase 1 multicenter, open-label, non-randomized trial with a classic 3+3 dose escalation and an expansion cohort in patients with unresectable HCC, Child Pugh A, ECOG 0 or 1. Two initial cohorts consisted of I.V. administration of Tirapazamine followed by superselective TAE while the remaining three cohorts underwent intraarterial administration of Tirapazamine with superselective TAE. Safety and tolerability were assessed using NCI CTCAE 4.0 with clinical, imaging and laboratory examinations including pharmacokinetic (PK) analysis and an electrocardiogram 1 day pre-dose, at 1, 2, 4, 6, 10, and 24 hours post-TPZ infusion and an additional PK at 15- and 30-minutes post-TPZ. Tumor responses were evaluated using mRECIST criteria.
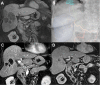
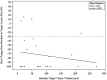
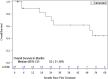
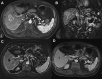
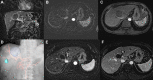
Results: Twenty-seven patients (mean [range] age of 66.4 [37-79] years) with unresectable HCC were enrolled between July 2015 and January 2018. Two patients were lost to follow-up. Mean tumor size was 6.53 cm ± 2.60 cm with a median of two lesions per patient. Dose limiting toxicity and maximum tolerated dose were not reached. The maximal TPZ dose was 10 mg/m2 I.V. and 20 mg/m2 I.A. One adverse event (AE) was reported in all patients with fatigue, decreased appetite or pain being most common. Grade 3-5 AE were hypertension and transient elevation of AST/ALT in 70.4% of patients. No serious AE were drug related. Sixty percent (95% CI=38.7-78.9) achieved complete response (CR), and 84% (95% CI=63.9-95.5) had complete and partial response per mRECIST for target lesions.
Discussion: TAE with TPZ was safe and tolerable with encouraging results justifying pursuit of a Phase II trial.
Keywords: hepatocellular carcinoma; hypoxia activated agent; image guided locoregional therapies; phase I trial; transarterial chemoembolization.
© 2021 Abi-Jaoudeh et al.
Conflict of interest statement
Dr Abi-Jaoudeh is principal investigator on a research agreement between the University of California Irvine and Teclison Inc, Philips Medical Systems Inc. Dr. Abi-Jaoudeh is principal investigator on sponsored research by Sillajen Inc., SIRTEX Inc., Dr. Abi-Jaoudeh has shares in Bruin Biosciences Inc and has served on an advisory board with Genentech, Medtronic, Eisai, and QED therapeutics Inc. Dr. Dayyani was paid by the speaker’s bureau of Amgen, Eisai, Exelixis, Taiho, Merck, BMS, Deciphera, Signatera, Ipsen, and Sirtex. He is a consultant for Eisai, Array, Exelixis, Genentech, Foundation Medicine, and Ipsen. Dr. Fernando, Dr. Hwang, Dr Liang, and, Dr. Javan have not declared any conflicts of interest. Dr. Fidelman has reported grants from Merck, SIRTEX and Boston Scientific. Dr Chen has declared grants from Bristol Myers Squibb and personal fees from Roche. Dr. Imagawa has shares and is part owner of Bruin Biosciences Inc., is consultant for Bayer Inc, and is principal investigator on sponsored research by Beigene and Eisai. He is also paid by the speaker’s bureau of Eisai.
Figures



Similar articles
-
Tirapazamine-loaded CalliSpheres microspheres enhance synergy between tirapazamine and embolization against liver cancer in an animal model.Biomed Pharmacother. 2022 Jul;151:113123. doi: 10.1016/j.biopha.2022.113123. Epub 2022 May 17. Biomed Pharmacother. 2022. PMID: 35594702
-
Tirapazamine-loaded CalliSpheres microspheres: Preparation and characterization as a chemoembolization agent for liver cancer.MethodsX. 2023 Apr 16;10:102188. doi: 10.1016/j.mex.2023.102188. eCollection 2023. MethodsX. 2023. PMID: 37168773 Free PMC article.
-
A rat toxicological study of intra-arterial injection of Tirapazamine, a hypoxia-activating Cancer therapeutic agent, followed by hepatic artery ligation.Invest New Drugs. 2021 Jun;39(3):747-755. doi: 10.1007/s10637-020-01057-3. Epub 2021 Jan 11. Invest New Drugs. 2021. PMID: 33428079
-
Transarterial (chemo)embolisation versus no intervention or placebo for liver metastases.Cochrane Database Syst Rev. 2020 Mar 12;3(3):CD009498. doi: 10.1002/14651858.CD009498.pub4. Cochrane Database Syst Rev. 2020. PMID: 32163181 Free PMC article.
-
Transarterial Therapies for Hepatocellular Carcinoma.Liver Cancer. 2016 Nov;6(1):27-33. doi: 10.1159/000449347. Epub 2016 Nov 29. Liver Cancer. 2016. PMID: 27995085 Free PMC article. Review.
Cited by
-
Hypoxia signaling in hepatocellular carcinoma: Challenges and therapeutic opportunities.Cancer Metastasis Rev. 2023 Sep;42(3):741-764. doi: 10.1007/s10555-022-10071-1. Epub 2022 Dec 22. Cancer Metastasis Rev. 2023. PMID: 36547748 Review.
-
Hypoxia as a Target for Combination with Transarterial Chemoembolization in Hepatocellular Carcinoma.Pharmaceuticals (Basel). 2024 Aug 11;17(8):1057. doi: 10.3390/ph17081057. Pharmaceuticals (Basel). 2024. PMID: 39204162 Free PMC article. Review.
-
Drug-Eluting Embolic Loaded with Tyrosine Kinase Inhibitor Targeted Therapies for Transarterial Chemoembolization in a VX2 Model.Cancers (Basel). 2023 Jun 18;15(12):3236. doi: 10.3390/cancers15123236. Cancers (Basel). 2023. PMID: 37370846 Free PMC article.
-
Targeting Hypoxia-Inducible Factor-1α for the Management of Hepatocellular Carcinoma.Cancers (Basel). 2023 May 12;15(10):2738. doi: 10.3390/cancers15102738. Cancers (Basel). 2023. PMID: 37345074 Free PMC article. Review.
-
Limitations of Fluorine 18 Fluoromisonidazole in Assessing Treatment-induced Tissue Hypoxia after Transcatheter Arterial Embolization of Hepatocellular Carcinoma: A Prospective Pilot Study.Radiol Imaging Cancer. 2022 May;4(3):e210094. doi: 10.1148/rycan.210094. Radiol Imaging Cancer. 2022. PMID: 35485937 Free PMC article. Clinical Trial.
References
-
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

