Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members
- PMID: 34041295
- PMCID: PMC8012818
- DOI: 10.1183/23120541.00141-2021
Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members
Abstract
Background: It remains unknown whether and to what extent members of online "long COVID" peer support groups remain symptomatic and limited over time. Therefore, we aimed to evaluate symptoms in members of online long COVID peer support groups up to 6 months after the onset of coronavirus disease 2019 (COVID-19)-related symptoms.
Methods: Demographics, symptoms, health status, work productivity, functional status and health-related quality of life were assessed about 3 and 6 months after the onset of COVID-19-related symptoms in members of online long COVID peer support groups.
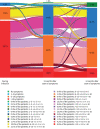
Results: Data from 239 patients with a confirmed COVID-19 diagnosis (83% women; median (interquartile range) age 50 (39-56) years) were analysed. During the infection, a median (interquartile range) of 15 (11-18) symptoms was reported, which was significantly lower 3 and 6 months later: 6 (4-9) and 6 (3-8), respectively (p<0.05). From 3 to 6 months follow-up, the proportion of patients without symptoms increased from 1.3% to only 5.4% (p<0.001). Patients also reported a significantly improved work productivity (work absenteeism and presenteeism: 73% versus 52% and 66% versus 60%, respectively), self-reported good health (9.2% versus 16.7%), functional status (mean±sd Post-COVID-19 Functional Status scale: 2.4±0.9 versus 2.2±1.0) and health-related quality of life (all p<0.05).
Conclusion: Although patients with confirmed COVID-19, who were all members of online long COVID peer support groups, reported significant improvements in work productivity, functional status and quality of life between 3 and 6 months follow-up, these data clearly highlight the long-term impact of COVID-19, as approximately 6 months after the onset of COVID-19-related symptoms a large proportion still experienced persistent symptoms, a moderate-to-poor health, moderate-to-severe functional limitations, considerable loss in work productivity, and/or an impaired quality of life. Action is needed to improve the management and healthcare of these patients.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: A.W. Vaes has nothing to disclose. Conflict of interest: Y.M.J. Goërtz has nothing to disclose. Conflict of interest: M. Van Herck has nothing to disclose. Conflict of interest: F.V.C. Machado has nothing to disclose. Conflict of interest: R. Meys has nothing to disclose. Conflict of interest: J.M. Delbressine has nothing to disclose. Conflict of interest: S. Houben-Wilke has nothing to disclose. Conflict of interest: S. Gaffron has nothing to disclose. Conflict of interest: D. Maier reports Biomax provides data management and analysis services to CIRO. Conflict of interest: C. Burtin has nothing to disclose. Conflict of interest: R. Posthuma has nothing to disclose. Conflict of interest: N.P.H. van Loon has nothing to disclose. Conflict of interest: F.M.E. Franssen reports grants and personal fees from AstraZeneca, and personal fees from Boehringer Ingelheim, Chiesi, GSK and TEVA, outside the submitted work. Conflict of interest: B. Hajian has nothing to disclose. Conflict of interest: S.O. Simons reports personal fees from AstraZeneca and grants from GSK, outside the submitted work. Conflict of interest: J.F.M. Van Boven has nothing to disclose. Conflict of interest: F.A. Klok reports grants from Bayer, Bristol Meyer Squibb, Boehringer Ingelheim, MSD, Daiichi-Sankyo, Actelion, the Dutch Thrombosis Association, the Dutch Heart Foundation, and the Netherlands Organisation for Health Research and Development, outside the submitted work. Conflict of interest: B. Spaetgens has nothing to disclose. Conflict of interest: C.M.H. Pinxt has nothing to disclose. Conflict of interest: L.Y.L. Liu has nothing to disclose. Conflict of interest: G. Wesseling has nothing to disclose. Conflict of interest: Y. Spies has nothing to disclose. Conflict of interest: H. Vijlbrief has nothing to disclose. Conflict of interest: A.J. Van ’t Hul has nothing to disclose. Conflict of interest: D.J.A. Janssen reports personal fees from AstraZeneca, Boehringer Ingelheim and Novartis, outside the submitted work. Conflict of interest: M.A. Spruit reports grants from Lung Foundation Netherlands and Stichting Astma Bestrijding, and grants and personal fees from AstraZeneca and Boehringer Ingelheim, outside the submitted work.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
