Determination of singlet oxygen quenching rate and mechanism of γ-oryzanol
- PMID: 34041405
- PMCID: PMC8141896
- DOI: 10.1016/j.heliyon.2021.e07065
Determination of singlet oxygen quenching rate and mechanism of γ-oryzanol
Abstract
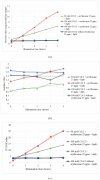
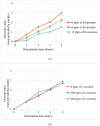
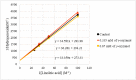
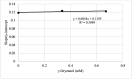
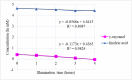
Photooxidation is one of the causes of quality deterioration in food. An antioxidant or singlet oxygen quencher is urgently needed to prevent photooxidation. γ-Oryzanol was recognized as a naturally present antioxidant in rice bran products. This research aimed to calculate the singlet oxygen quenching rate and its mechanism of γ-oryzanol to evaluate the potency of γ-oryzanol as singlet oxygen quencher. A series of linoleic acid (50 and 100 mM) or γ-oryzanol (0.7 and 1.5 mM) were prepared separately in ethanol: chloroform (96:4, v/v) containing 25 ppm of erythrosine. High-Performance Liquid Chromatography quantified the degradation of γ-oryzanol. Meanwhile, Gas Chromatography determined the changes in linoleic acid content during photooxidation. The singlet oxygen quenching rate was calculated by steady-state. The singlet oxygen quenching rate of γ-oryzanol was 3.04 × 106/M/s by physical and chemical quenching mechanism. Photooxidation caused the declined of γ-oryzanol by 0.1421 mM/h. Based on singlet oxygen quenching rate calculation, it suggests that γ-oryzanol can perform as a singlet oxygen quencher with slightly dominated by physical quenching mechanism (52.28%). The rest it performed via a chemical quenching mechanism.
Keywords: Nanoemulsion; Photooxidation; Rice bran oil; Singlet oxygen quenching; γ-Oryzanol.
© 2021 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Kinetic study of the quenching reaction of singlet oxygen by seven rice bran extracts in ethanol solution. Development of a singlet oxygen absorption capacity (SOAC) assay method.Biosci Biotechnol Biochem. 2015;79(12):2063-72. doi: 10.1080/09168451.2015.1069701. Epub 2015 Jul 29. Biosci Biotechnol Biochem. 2015. PMID: 26222314
-
Determination of γ-Oryzanol in Refined Rice Bran Oil by Nuclear Magnetic Resonance Method.J Nutr Sci Vitaminol (Tokyo). 2019;65(Supplement):S72-S74. doi: 10.3177/jnsv.65.S72. J Nutr Sci Vitaminol (Tokyo). 2019. PMID: 31619651
-
Simultaneous Determination of Vitamin E and γ-Oryzanol in Rice Bran Oil via HPSEC-PDA without Sample Pretreatment.J Oleo Sci. 2023;72(7):655-665. doi: 10.5650/jos.ess22257. J Oleo Sci. 2023. PMID: 37380482
-
Biological and Pharmacological Effects of Gamma-oryzanol: An Updated Review of the Molecular Mechanisms.Curr Pharm Des. 2021;27(19):2299-2316. doi: 10.2174/1381612826666201102101428. Curr Pharm Des. 2021. PMID: 33138751 Review.
-
[Gamma-oryzanol: an important component in rice brain oil].Arch Latinoam Nutr. 1998 Mar;48(1):7-12. Arch Latinoam Nutr. 1998. PMID: 9754398 Review. Spanish.
Cited by
-
Singlet Oxygen in Food: A Review on Its Formation, Oxidative Damages, Quenchers, and Applications in Preservation.Antioxidants (Basel). 2025 Jul 15;14(7):865. doi: 10.3390/antiox14070865. Antioxidants (Basel). 2025. PMID: 40722969 Free PMC article. Review.
References
-
- AOCS . fifth ed. American Oil Chemists Society; IL: 2004. Official Methods and Recommended Practices of the AOCS.
-
- Ariviani S., Raharjo S., Hastuti P. Potensi mikroemulsi β -karoten dalam menghambat fotooksidasi vitamin C sistem aqueous. Jurnal Teknologi dan Industri Pangan. 2011;XXII(1):33–39.
-
- Choe E., Min D.B. Chemistry and reactions of reactive oxygen species in foods. J. Food Sci. 2005;70(9):142–159. - PubMed
-
- Cuevas M.S., Souza P.T.D., Christianne E., Rodrigues C., Meirelles A.J.A. Quantification and determination of composition of steryl ferulates in refined rice bran oils using an UPLC - MS method. JAOCS (J. Am. Oil Chem. Soc.) 2017;943:375–385.
-
- Dhavamani S., Poorna Y., Rao C., Lokesh B.R. Total antioxidant activity of selected vegetable oils and their influence on total antioxidant values in vivo : a photochemiluminescence based analysis. Food Chem. 2014;164:551–555. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

