Discovery, affinity maturation and multimerization of small molecule ligands against human tyrosinase and tyrosinase-related protein 1
- PMID: 34041485
- PMCID: PMC8130610
- DOI: 10.1039/d0md00310g
Discovery, affinity maturation and multimerization of small molecule ligands against human tyrosinase and tyrosinase-related protein 1
Abstract
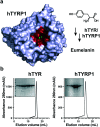
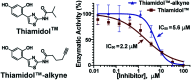
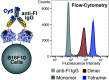
Human tyrosinase (hTYR) and tyrosinase-related protein 1 (hTYRP1) are closely-related enzymes involved in the synthesis of melanin, which are selectively expressed in melanocytes and, in a pathological context, in melanoma lesions. We used a previously described tyrosinase inhibitor (Thiamidol™) and DNA-encoded library technology for the discovery of novel hTYR and hTYRP1 ligands, that could be used as vehicles for melanoma targeting. Performing de novo selections with DNA-encoded libraries, we discovered novel ligands capable of binding to both hTYR and hTYRP1. More potent ligands were obtained by multimerizing Thiamidol™ moieties, leading to homotetrameric structures that avidly bound to melanoma cells, as revealed by flow cytometry. These findings suggest that melanoma lesions may, in the future, be targeted not only by monoclonal antibody reagents but also by small organic ligands.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare the following competing financial interest(s): D. N. is a cofounder and shareholder of Philochem AG.
Figures



Similar articles
-
Structure-Based Virtual Screening of Furan-1,3,4-Oxadiazole Tethered N-phenylacetamide Derivatives as Novel Class of hTYR and hTYRP1 Inhibitors.Pharmaceuticals (Basel). 2023 Feb 23;16(3):344. doi: 10.3390/ph16030344. Pharmaceuticals (Basel). 2023. PMID: 36986444 Free PMC article.
-
Computational methods to analyze and predict the binding mode of inhibitors targeting both human and mushroom tyrosinase.Eur J Med Chem. 2023 Nov 15;260:115771. doi: 10.1016/j.ejmech.2023.115771. Epub 2023 Aug 28. Eur J Med Chem. 2023. PMID: 37657271
-
Modified vaccinia virus Ankara for delivery of human tyrosinase as melanoma-associated antigen: induction of tyrosinase- and melanoma-specific human leukocyte antigen A*0201-restricted cytotoxic T cells in vitro and in vivo.Cancer Res. 1999 Oct 1;59(19):4955-63. Cancer Res. 1999. PMID: 10519409
-
Characterization of melanosome-associated proteins by establishment of monoclonal antibodies and immunoscreening of a melanoma cDNA library through an anti-melanosome antibody.Melanoma Res. 1993 Oct;3(5):331-6. doi: 10.1097/00008390-199310000-00005. Melanoma Res. 1993. PMID: 8292889 Review.
-
Advances in fluorescent probes for detection and imaging of endogenous tyrosinase activity.Anal Biochem. 2020 Apr 1;594:113614. doi: 10.1016/j.ab.2020.113614. Epub 2020 Feb 7. Anal Biochem. 2020. PMID: 32035843 Review.
Cited by
-
Recent advances in DNA-encoded dynamic libraries.RSC Chem Biol. 2022 Feb 17;3(4):407-419. doi: 10.1039/d2cb00007e. eCollection 2022 Apr 6. RSC Chem Biol. 2022. PMID: 35441147 Free PMC article. Review.
-
Protein-templated ligand discovery via the selection of DNA-encoded dynamic libraries.Nat Chem. 2024 Apr;16(4):543-555. doi: 10.1038/s41557-024-01442-y. Epub 2024 Feb 7. Nat Chem. 2024. PMID: 38326646
-
Heterocyclic Compounds as Synthetic Tyrosinase Inhibitors: Recent Advances.Int J Mol Sci. 2023 May 22;24(10):9097. doi: 10.3390/ijms24109097. Int J Mol Sci. 2023. PMID: 37240442 Free PMC article. Review.
-
Control of the antitumour activity and specificity of CAR T cells via organic adapters covalently tethering the CAR to tumour cells.Nat Biomed Eng. 2024 May;8(5):529-543. doi: 10.1038/s41551-023-01102-5. Epub 2023 Oct 5. Nat Biomed Eng. 2024. PMID: 37798444
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

