Neurotrophic effects of Botulinum neurotoxin type A in hippocampal neurons involve activation of Rac1 by the non-catalytic heavy chain (HCC/A)
- PMID: 34041508
- PMCID: PMC8143998
- DOI: 10.1016/j.ibneur.2021.04.002
Neurotrophic effects of Botulinum neurotoxin type A in hippocampal neurons involve activation of Rac1 by the non-catalytic heavy chain (HCC/A)
Abstract
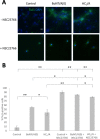
Botulinum neurotoxins (BoNTs) are extremely potent naturally occurring poisons that act by silencing neurotransmission. Intriguingly, in addition to preventing presynaptic vesicle fusion, BoNT serotype A (BoNT/A) can also promote axonal regeneration in preclinical models. Here we report that the non-toxic C-terminal region of the receptor-binding domain of heavy chain BoNT/A (HCC/A) activates the small GTPase Rac1 and ERK pathway to potentiate axonal outgrowth, dendritic protrusion formation and synaptic vesicle release in hippocampal neurons. These data are consistent with HCC/A exerting neurotrophic properties, at least in part, independent of any BoNT catalytic activity or toxic effect.
Keywords: Botulinum neurotoxin; ERK; Hippocampal neuron; Neurotrophy; Rac1.
© 2021 The Authors.
Conflict of interest statement
LSV received a collaborative scholarship from Ipsen to fund his PhD. None of the workers at Ipsen participated in the design, performance or analysis of the experiments, or in writing the manuscript, but they did provide facilities and reagents necessary for completion of the work.
Figures

References
-
- Agthong S., Koonam J., Kaewsema A., Chentanez V. Inhibition of MAPK ERK impairs axonal regeneration without an effect on neuronal loss after nerve injury. Neurol. Res. 2009;31:1068–1074. - PubMed
-
- Alvarez Julia A., Frasch A.C., Fuchsova B. Neuronal filopodium formation induced by the membrane glycoprotein M6a (Gpm6a) is facilitated by coronin-1a, Rac1, and p21-activated kinase 1 (Pak1) J. Neurochem. 2016;137:46–61. - PubMed
-
- Angaut-Petit D., Molgo J., Comella J.X., Faille L., Tabti N. Terminal sprouting in mouse neuromuscular junctions poisoned with botulinum type A toxin: morphological and electrophysiological features. Neuroscience. 1990;37:799–808. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
