Efficacy and safety of short-wave diathermy treatment for moderate COVID-19 patients: a prospective, double-blind, randomized controlled clinical study
- PMID: 34042412
- PMCID: PMC9980486
- DOI: 10.23736/S1973-9087.21.06892-1
Efficacy and safety of short-wave diathermy treatment for moderate COVID-19 patients: a prospective, double-blind, randomized controlled clinical study
Abstract
Background: Millions of human beings have suffered in the epidemic of Coronavirus disease 2019 (COVID-19), but until now the effective treatment methods have been limited.
Aim: This study aimed to evaluate the efficacy and safety of short-wave diathermy (SWD) treatment for moderate COVID-19 patients.
Design: A prospective, double-blind, randomized controlled clinical study.
Setting: Inpatients Unit of a COVID-19 specialized hospital.
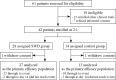
Population: Forty-two patients with moderate COVID-19 were randomly allocated at a 2:1 ratio to two groups: the SWD group and the control group.
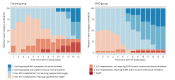
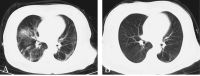
Methods: Participants of the SWD group received SWD treatment, and participants of the control group received placebo SWD treatment for one session per day, 10 minutes per session, for no more than 14 days. Both groups were given standard care treatment. Primary outcome was the rate of clinical improvement according to a seven-category ordinal scale. Secondary outcomes included the rate of computed tomography (CT) improvement and the rate of potential adverse events.
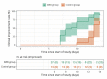
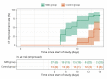
Results: Clinical improvement occurred in 92.6% of patients in the SWD group by day 14 compared with 69.2% of patients in the control group (P=0.001). The Cox model indicated that the SWD group had a higher clinical improvement probability than the control group (hazard ratio: 3.045; 95% CI: 1.391-6.666; P=0.005). Similarly, CT improvement occurred in 85.2% of patients in the SWD group and 46.2% of patients in the control group respectively by day 14 (P=0.001). The Cox model indicated SWD group had a higher CT improvement probability than control group (hazard ratio: 3.720; 95% CI: 1.486-9.311; P=0.005). There was no significant difference in adverse events between the SWD group and the control group (2 of 27 [7.4%] SWD vs. 1 of 13 [7.7%] control, P=1.000), the most frequent of which were headache (1 of 27 [3.7%] SWD vs. 1 of 13 [7.7%] control patients) and dizziness (1 of 27 [3.7%] SWD vs. 0 of 13 [0%] control patients).
Conclusions: SWD is a valid and reliable adjuvant therapy with a favorable safety profile for moderate COVID-19 patients.
Clinical rehabilitation impact: Clinically relevant information is lacking regarding the efficacy and safety of SWD for patients with COVID-19. This study provides the first evidence that SWD is a promising adjuvant therapy for COVID-19.
Conflict of interest statement
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
The curative effects of shortwave diathermy on treating Novel coronavirus (COVID-19) pneumonia: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jul 3;21(1):609. doi: 10.1186/s13063-020-04534-5. Trials. 2020. PMID: 32620144 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
-
Frontier Breakthroughs: A Comprehensive Review of Diathermy in Dentistry With a Focus on Oral Medicine.Cureus. 2024 Apr 1;16(4):e57427. doi: 10.7759/cureus.57427. eCollection 2024 Apr. Cureus. 2024. PMID: 38699109 Free PMC article. Review.
Cited by
-
Rehabilitation and COVID-19: systematic review by Cochrane Rehabilitation.Eur J Phys Rehabil Med. 2023 Dec;59(6):800-818. doi: 10.23736/S1973-9087.23.08331-4. Eur J Phys Rehabil Med. 2023. PMID: 38214047 Free PMC article.
-
Endothelial Dysfunction in COVID-19: A Unifying Mechanism and a Potential Therapeutic Target.Biomedicines. 2022 Mar 30;10(4):812. doi: 10.3390/biomedicines10040812. Biomedicines. 2022. PMID: 35453563 Free PMC article. Review.
-
Regional moderate hyperthermia for mild-to-moderate COVID-19 (TherMoCoV study): a randomized controlled trial.Front Med (Lausanne). 2023 Dec 22;10:1256197. doi: 10.3389/fmed.2023.1256197. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38188344 Free PMC article.
-
The Efficacy of Electromagnetic Diathermy for the Treatment of Musculoskeletal Disorders: A Systematic Review with Meta-Analysis.J Clin Med. 2023 Jun 9;12(12):3956. doi: 10.3390/jcm12123956. J Clin Med. 2023. PMID: 37373650 Free PMC article. Review.
References
-
- Baricich A, Borg MB, Cuneo D, Cadario E, Azzolina D, Balbo PE, et al. No-more Covid Group . Midterm functional sequelae and implications in rehabilitation after COVID-19: a cross-sectional study. Eur J Phys Rehabil Med 2021;57:199–207. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.23736/S1973-9087.21.06699-5 - DOI - PubMed
-
- Hirayama A, Masui J, Murayama A, Fujita S, Okamoto J, Tanaka J, et al. The characteristics and clinical course of patients with COVID-19 who received invasive mechanical ventilation in Osaka, Japan. Int J Infect Dis 2021;102:282–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1016/j.ijid.2020.10.051 - DOI - PMC - PubMed
-
- Ugurov P, Popevski D, Gramosli T, Neziri D, Vuckova D, Gjorgon M, et al. Early Initiation of Extracorporeal Blood Purification Using the AN69ST (oXiris®) Hemofilter as a Treatment Modality for COVID-19 Patients: a Single-Centre Case Series. Rev Bras Cir Cardiovasc 2020. [Epub ahead of print] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.21470/1678-9741-2020-0403 - DOI - PMC - PubMed
-
- Liu W, Zhou P, Chen K, Ye Z, Liu F, Li X, et al. Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis. CMAJ 2020;192:E734–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1503/cmaj.200647 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

