Metformin effects on brain development following cranial irradiation in a mouse model
- PMID: 34042964
- PMCID: PMC8408860
- DOI: 10.1093/neuonc/noab131
Metformin effects on brain development following cranial irradiation in a mouse model
Abstract
Background: Cranial radiation therapy (CRT) is a mainstay of treatment for malignant pediatric brain tumors and high-risk leukemia. Although CRT improves survival, it has been shown to disrupt normal brain development and result in cognitive impairments in cancer survivors. Animal studies suggest that there is potential to promote brain recovery after injury using metformin. Our aim was to evaluate whether metformin can restore brain volume outcomes in a mouse model of CRT.
Methods: C57BL/6J mice were irradiated with a whole-brain radiation dose of 7 Gy during infancy. Two weeks of metformin treatment started either on the day of or 3 days after irradiation. In vivo magnetic resonance imaging was performed prior to irradiation and at 3 subsequent time points to evaluate the effects of radiation and metformin on brain development.
Results: Widespread volume loss in the irradiated brain appeared within 1 week of irradiation with limited subsequent recovery in volume outcomes. In many structures, metformin administration starting on the day of irradiation exacerbated radiation-induced injury, particularly in male mice. Metformin treatment starting 3 days after irradiation improved brain volume outcomes in subcortical regions, the olfactory bulbs, and structures of the brainstem and cerebellum.
Conclusions: Our results show that metformin treatment has the potential to improve neuroanatomical outcomes after CRT. However, both timing of metformin administration and subject sex affect structure outcomes, and metformin may also be deleterious. Our results highlight important considerations in determining the potential benefits of metformin treatment after CRT and emphasize the need for caution in repurposing metformin in clinical studies.
Keywords: MRI; metformin; neurodevelopment; neurogenesis; radiation.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



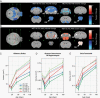
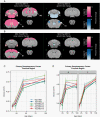



Similar articles
-
Exercise promotes growth and rescues volume deficits in the hippocampus after cranial radiation in young mice.NMR Biomed. 2023 Dec;36(12):e5015. doi: 10.1002/nbm.5015. Epub 2023 Aug 7. NMR Biomed. 2023. PMID: 37548099
-
p53 Loss Mitigates Early Volume Deficits in the Brains of Irradiated Young Mice.Int J Radiat Oncol Biol Phys. 2019 Feb 1;103(2):511-520. doi: 10.1016/j.ijrobp.2018.09.014. Epub 2018 Sep 20. Int J Radiat Oncol Biol Phys. 2019. PMID: 30243572
-
Spatiotemporal Mapping of Early Volume Loss in the Mouse Brain after Cranial Irradiation.Radiat Res. 2021 Oct 1;196(4):394-403. doi: 10.1667/RADE-21-00013.1. Radiat Res. 2021. PMID: 34270782
-
Long-Term Effect of Cranial Radiotherapy on Pituitary-Hypothalamus Area in Childhood Acute Lymphoblastic Leukemia Survivors.Curr Treat Options Oncol. 2016 Sep;17(9):50. doi: 10.1007/s11864-016-0426-0. Curr Treat Options Oncol. 2016. PMID: 27476159 Free PMC article. Review.
-
Neurocognitive function after radiotherapy for paediatric brain tumours.Nat Rev Neurol. 2012 Oct;8(10):578-88. doi: 10.1038/nrneurol.2012.182. Epub 2012 Sep 11. Nat Rev Neurol. 2012. PMID: 22964509 Review.
Cited by
-
Multimodal imaging with magnetization transfer and diffusion tensor imaging reveals evidence of myelin damage in children and youth treated for a brain tumor.Neurooncol Pract. 2024 Jan 19;11(3):307-318. doi: 10.1093/nop/npae003. eCollection 2024 Jun. Neurooncol Pract. 2024. PMID: 38737604 Free PMC article.
-
Brains in space: impact of microgravity and cosmic radiation on the CNS during space exploration.Nat Rev Neurosci. 2025 Jun;26(6):354-371. doi: 10.1038/s41583-025-00923-4. Epub 2025 Apr 17. Nat Rev Neurosci. 2025. PMID: 40247135 Review.
-
Effects of radiotherapy on the hippocampus and hippocampal neurogenesis: a systematic review of preclinical studies.Strahlenther Onkol. 2025 Apr;201(4):383-397. doi: 10.1007/s00066-024-02341-4. Epub 2025 Jan 12. Strahlenther Onkol. 2025. PMID: 39800777
-
Sex chromosomes and hormones independently influence healthy brain development but act similarly after cranial radiation.Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2404042121. doi: 10.1073/pnas.2404042121. Epub 2024 Aug 29. Proc Natl Acad Sci U S A. 2024. PMID: 39207735 Free PMC article.
-
Neuroprotective and Cardiometabolic Role of Vitamin E: Alleviating Neuroinflammation and Metabolic Disturbance Induced by AlCl3 in Rat Models.Biomedicines. 2023 Sep 4;11(9):2453. doi: 10.3390/biomedicines11092453. Biomedicines. 2023. PMID: 37760893 Free PMC article.
References
-
- Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22(4):706–713. - PubMed
-
- Scantlebury N, Bouffet E, Laughlin S, et al. . White matter and information processing speed following treatment with cranial-spinal radiation for pediatric brain tumor. Neuropsychology. 2016;30(4):425–438. - PubMed
-
- Padovani L, André N, Constine LS, Muracciole X. Neurocognitive function after radiotherapy for paediatric brain tumours. Nat Rev Neurol. 2012;8(10):578–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

