Evaluation of two different transarterial chemoembolization protocols using Lipiodol and degradable starch microspheres in therapy of hepatocellular carcinoma: a prospective trial
- PMID: 34043158
- PMCID: PMC8286929
- DOI: 10.1007/s12072-021-10193-8
Evaluation of two different transarterial chemoembolization protocols using Lipiodol and degradable starch microspheres in therapy of hepatocellular carcinoma: a prospective trial
Abstract
Background: This prospective randomized trial is designed to compare the performance of conventional transarterial chemoembolization (cTACE) using Lipiodol-only with additional use of degradable starch microspheres (DSM) for hepatocellular carcinoma (HCC) in BCLC-stage-B based on metric tumor response.
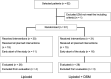
Methods: Sixty-one patients (44 men; 17 women; range 44-85) with HCC were evaluated in this IRB-approved HIPPA compliant study. The treatment protocol included three TACE-sessions in 4-week intervals, in all cases with Mitomycin C as a chemotherapeutic agent. Multiparametric magnetic resonance imaging (MRI) was performed prior to the first and 4 weeks after the last TACE. Two treatment groups were determined using a randomization sheet: In 30 patients, TACE was performed using Lipiodol only (group 1). In 31 cases Lipiodol was combined with DSMs (group 2). Response according to tumor volume, diameter, mRECIST criteria, and the development of necrotic areas were analyzed and compared using the Mann-Whitney-U, Kruskal-Wallis-H-test, and Spearman-Rho. Survival data were analyzed using the Kaplan-Meier estimator.
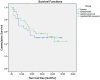
Results: A mean overall tumor volume reduction of 21.45% (± 62.34%) was observed with an average tumor volume reduction of 19.95% in group 1 vs. 22.95% in group 2 (p = 0.653). Mean diameter reduction was measured with 6.26% (± 34.75%), for group 1 with 11.86% vs. 4.06% in group 2 (p = 0.678). Regarding mRECIST criteria, group 1 versus group 2 showed complete response in 0 versus 3 cases, partial response in 2 versus 7 cases, stable disease in 21 versus 17 cases, and progressive disease in 3 versus 1 cases (p = 0.010). Estimated overall survival was in mean 33.4 months (95% CI 25.5-41.4) for cTACE with Lipiosol plus DSM, and 32.5 months (95% CI 26.6-38.4), for cTACE with Lipiodol-only (p = 0.844), respectively.
Conclusions: The additional application of DSM during cTACE showed a significant benefit in tumor response according to mRECIST compared to cTACE with Lipiodol-only. No benefit in survival time was observed.
Keywords: DSM; Hepatocellular carcinoma; Lipiodol; Magnetic resonance imaging; Mitomycin C; Necrotic area; Response; Survival; TACE; Tumor volume.
© 2021. The Author(s).
Conflict of interest statement
Thomas J. Vogl, Marcel C. Langenbach, Renate Hammerstingl, Moritz H. Albrecht, AR. Chatterjee, Tatjana Gruber-Rouh declare that they have no conflict of interest.
Figures

Similar articles
-
Transarterial chemoembolization (TACE) using mitomycin and lipiodol with or without degradable starch microspheres for hepatocellular carcinoma: comparative study.BMC Cancer. 2018 Feb 14;18(1):188. doi: 10.1186/s12885-018-4099-x. BMC Cancer. 2018. PMID: 29444653 Free PMC article.
-
Transarterial chemoembolization of colorectal cancer liver metastasis: improved tumor response by DSM-TACE versus conventional TACE, a prospective, randomized, single-center trial.Eur Radiol. 2021 Apr;31(4):2242-2251. doi: 10.1007/s00330-020-07253-2. Epub 2020 Sep 22. Eur Radiol. 2021. PMID: 32960329 Clinical Trial.
-
Degradable starch microspheres versus ethiodol and doxorubicin in transarterial chemoembolization of hepatocellular carcinoma.J Vasc Interv Radiol. 2014 Feb;25(2):240-7. doi: 10.1016/j.jvir.2013.10.007. Epub 2013 Nov 28. J Vasc Interv Radiol. 2014. PMID: 24291001
-
A Review Of Mri Appearances Of Lipiodol In Conventional Tace (Ctace) Treated Hepatocellular Carcinomas.J Ayub Med Coll Abbottabad. 2023 Oct-Dec;35(Suppl 1)(4):S740-S745. doi: 10.55519/JAMC-S4-11966. J Ayub Med Coll Abbottabad. 2023. PMID: 38406903 Review.
-
Initiative on Superselective Conventional Transarterial Chemoembolization Results (INSPIRE).Cardiovasc Intervent Radiol. 2022 Oct;45(10):1430-1440. doi: 10.1007/s00270-022-03233-9. Epub 2022 Aug 17. Cardiovasc Intervent Radiol. 2022. PMID: 35978174 Free PMC article. Review.
Cited by
-
Temporary Flow Diversion in Oncological Embolization Procedures Using Degradable Starch Microspheres.Diagnostics (Basel). 2024 Dec 17;14(24):2844. doi: 10.3390/diagnostics14242844. Diagnostics (Basel). 2024. PMID: 39767205 Free PMC article.
-
Role of Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma.J Clin Transl Hepatol. 2023 Apr 28;11(2):480-489. doi: 10.14218/JCTH.2022.00293. Epub 2022 Sep 6. J Clin Transl Hepatol. 2023. PMID: 36643046 Free PMC article. Review.
-
Emerging Polymer Materials in Trackable Endovascular Embolization and Cell Delivery: From Hype to Hope.Biomimetics (Basel). 2022 Jun 10;7(2):77. doi: 10.3390/biomimetics7020077. Biomimetics (Basel). 2022. PMID: 35735593 Free PMC article. Review.
-
Biodegradable Microspheres for Transarterial Chemoembolization in Malignant Liver Disease.Medicina (Kaunas). 2024 Apr 22;60(4):678. doi: 10.3390/medicina60040678. Medicina (Kaunas). 2024. PMID: 38674324 Free PMC article. Review.
-
CXCL1 and CXCL6 Are Potential Predictors for HCC Response to TACE.Curr Oncol. 2023 Mar 20;30(3):3516-3528. doi: 10.3390/curroncol30030267. Curr Oncol. 2023. PMID: 36975480 Free PMC article.
References
-
- Liver EA for the S of the, Cancer EO for R and T of. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

