Use of contrast enhanced ultrasound in testicular diseases: A comprehensive review
- PMID: 34043256
- PMCID: PMC8640938
- DOI: 10.1111/andr.13057
Use of contrast enhanced ultrasound in testicular diseases: A comprehensive review
Abstract
Background: Contrast-enhanced ultrasound (CEUS) is a sonographic technique that increases the diagnostic accuracy of ultrasound and color Doppler ultrasound (CDUS) when studying testicular abnormalities. However, its role in clinical practice is still debatable because there are no accepted standards regarding how and when this technique should be used for patients with testicular disease.
Objectives: To perform a nonsystematic review of the current literature to highlight the strength and flaws of performing CEUS and to provide a critical overview of current research evidence on this topic.
Materials and methods: A thorough search of published peer-reviewed studies in PubMed was performed using proper keywords.
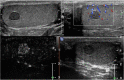
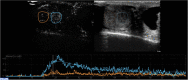
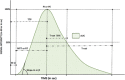
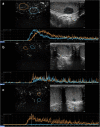
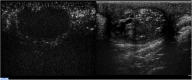
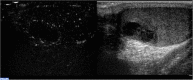
Results: Strong enhancement of neoplastic lesions (both benign and malignant) during CEUS aids in differential diagnosis with non-neoplastic lesions, which usually appears either nonenhanced or enhanced in a manner similar to that of the surrounding parenchyma. CEUS enhancement has a high predictive value in the identification of neoplastic lesions, whereas a similar or complete absence of enhancement may be interpreted as strong evidence of benignity, although there are exceptions. Literature on quantitative analysis is still scarce, though promising, particularly in distinguishing benign from malignant neoplasms. Furthermore, CEUS may be useful in many emergency situations, such as acute scrotum, blunt scrotal trauma, and focal infarction of the testis. Finally, CEUS can help increase the probability of sperm recovery in azoospermic males.
Discussion and conclusion: CEUS is a safe, easy-to-perform, and cost-effective diagnostic tool that can provide a more accurate diagnosis in testicular lesions and acute scrotal disease. However, further studies with larger cohorts are required to refine the differential diagnosis between benign and malignant neoplasms. Finally, these preliminary results can instigate the development of innovative research on pre-testicular sperm extraction to increase the chances of sperm recovery.
Keywords: CEUS; acute scrotum; infertility; testicular tumor; testis.
© 2021 American Society of Andrology and European Academy of Andrology.
Conflict of interest statement
The authors declare no conflict of interest regarding the publication of this article.
Figures



References
-
- Lotti F, Frizza F, Balercia G, et al. The European Academy of Andrology (EAA) ultrasound study on healthy, fertile men: scrotal ultrasound reference ranges and associations with clinical, seminal, and biochemical characteristics. Andrology. 2021;9(2):559–576. - PubMed
-
- Dieckmann KP, Frey U, Lock G. Contemporary diagnostic work‐up of testicular germ cell tumours. Nat Rev Urol. 2013;10(12):703–712. - PubMed
-
- Manganaro L, Saldari M, Pozza C, et al. Dynamic contrast‐enhanced and diffusion‐weighted MR imaging in the characterisation of small, non‐palpable solid testicular tumours. Eur Radiol. 2018;28(2):554–564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

