Structure-Activity Relationship of Fluorinated Sialic Acid Inhibitors for Bacterial Sialylation
- PMID: 34043338
- PMCID: PMC8382218
- DOI: 10.1021/acs.bioconjchem.1c00194
Structure-Activity Relationship of Fluorinated Sialic Acid Inhibitors for Bacterial Sialylation
Abstract
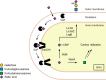
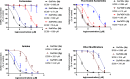
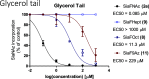
Bacterial pathogens such as Nontypeable Haemophilus influenzae (NTHi) can evade the immune system by taking up and presenting host-derived sialic acids. Herein, we report a detailed structure-activity relationship of sialic acid-based inhibitors that prevent the transfer of host sialic acids to NTHi. We report the synthesis and biological evaluation of C-5, C-8, and C-9 derivatives of the parent compound 3-fluorosialic acid (SiaNFAc). Small modifications are tolerated at the C-5 and C-9 positions, while the C-8 position does not allow for modification. These structure-activity relationships define the chemical space available to develop selective bacterial sialylation inhibitors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Severi E.; Randle G.; Kivlin P.; Whitfield K.; Young R.; Moxon R.; Kelly D.; Hood D.; Thomas G. H. (2005) Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter. Mol. Microbiol. 58, 1173–1185. 10.1111/j.1365-2958.2005.04901.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

