Advances in the Microbiology of Stenotrophomonas maltophilia
- PMID: 34043457
- PMCID: PMC8262804
- DOI: 10.1128/CMR.00030-19
Advances in the Microbiology of Stenotrophomonas maltophilia
Abstract
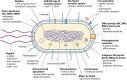
Stenotrophomonas maltophilia is an opportunistic pathogen of significant concern to susceptible patient populations. This pathogen can cause nosocomial and community-acquired respiratory and bloodstream infections and various other infections in humans. Sources include water, plant rhizospheres, animals, and foods. Studies of the genetic heterogeneity of S. maltophilia strains have identified several new genogroups and suggested adaptation of this pathogen to its habitats. The mechanisms used by S. maltophilia during pathogenesis continue to be uncovered and explored. S. maltophilia virulence factors include use of motility, biofilm formation, iron acquisition mechanisms, outer membrane components, protein secretion systems, extracellular enzymes, and antimicrobial resistance mechanisms. S. maltophilia is intrinsically drug resistant to an array of different antibiotics and uses a broad arsenal to protect itself against antimicrobials. Surveillance studies have recorded increases in drug resistance for S. maltophilia, prompting new strategies to be developed against this opportunist. The interactions of this environmental bacterium with other microorganisms are being elucidated. S. maltophilia and its products have applications in biotechnology, including agriculture, biocontrol, and bioremediation.
Keywords: S. maltophilia; Stenotrophomonas; antimicrobial agents; antimicrobial resistance; biofilms; biotechnology; cystic fibrosis; genomes; pathogenesis; risk factors.
Figures



References
-
- Gales AC, Castanheira M, Jones RN, Sader HS. 2012. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn Microbiol Infect Dis 73:354–360. 10.1016/j.diagmicrobio.2012.04.007. - DOI - PubMed
-
- Sader HS, Farrell DJ, Flamm RK, Jones RN. 2014. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009–2012. Int J Antimicrob Agents 43:328–334. 10.1016/j.ijantimicag.2014.01.007. - DOI - PubMed
-
- Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. 2019. Antimicrobial susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect Dis 6:S34–S46. 10.1093/ofid/ofy293. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

