The Abbott PanBio WHO emergency use listed, rapid, antigen-detecting point-of-care diagnostic test for SARS-CoV-2-Evaluation of the accuracy and ease-of-use
- PMID: 34043631
- PMCID: PMC8158996
- DOI: 10.1371/journal.pone.0247918
The Abbott PanBio WHO emergency use listed, rapid, antigen-detecting point-of-care diagnostic test for SARS-CoV-2-Evaluation of the accuracy and ease-of-use
Abstract
Objectives: Diagnostics are essential for controlling the pandemic. Identifying a reliable and fast diagnostic device is needed for effective testing. We assessed performance and ease-of-use of the Abbott PanBio antigen-detecting rapid diagnostic test (Ag-RDT).
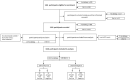
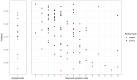
Methods: This prospective, multi-centre diagnostic accuracy study enrolled at two sites in Germany. Following routine testing with reverse-transcriptase polymerase chain reaction (RT-PCR), a second study-exclusive swab was performed for Ag-RDT testing. Routine swabs were nasopharyngeal (NP) or combined NP/oropharyngeal (OP) whereas the study-exclusive swabs were NP. To evaluate performance, sensitivity and specificity were assessed overall and in predefined sub-analyses accordingly to cycle-threshold values, days after symptom onset, disease severity and study site. Additionally, an ease-of-use assessment (EoU) and System Usability Scale (SUS) were performed.
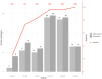
Results: 1108 participants were enrolled between Sept 28 and Oct 30, 2020. Of these, 106 (9.6%) were PCR-positive. The Abbott PanBio detected 92/106 PCR-positive participants with a sensitivity of 86.8% (95% CI: 79.0% - 92.0%) and a specificity of 99.9% (95% CI: 99.4%-100%). The sub-analyses indicated that sensitivity was 95.8% in Ct-values <25 and within the first seven days from symptom onset. The test was characterized as easy to use (SUS: 86/100) and considered suitable for point-of-care settings.
Conclusion: The Abbott PanBio Ag-RDT performs well for SARS-CoV-2 testing in this large manufacturer independent study, confirming its WHO recommendation for Emergency Use in settings with limited resources.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization. Director-General’s opening remarks at the media briefing on COVID-19: World Health Organization; 2020. [updated 16.03.202010.11.2020]. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
-
- World Health Organization. Antigendetection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays—Interim Guidance https://www.who.int/publications/i/item/antigen-detection-in-the-diagnos...; 2020. 11.09.2020.
-
- Foundation of Innovative New Diagnsotics. SARS-CoV-2 diagnostic pipeline: Foundation of Innovative New Diagnostics 2020. [https://www.finddx.org/covid-19/pipeline/?avance=all&type=Rapid+diagnost....
-
- Krüger LJ, Gaeddert M, Köppel L, Brümmer LE, Gottschalk C, Miranda IB, et al. Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. medRxiv. 2020:2020.
-
- World Health Organisation. WHO Emergency Use Listing for In vitro diagnostics (IVDs) Detecting SARS-CoV-2. 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous