Distinct responses of human peripheral blood cells to different misfolded protein oligomers
- PMID: 34043816
- PMCID: PMC8442237
- DOI: 10.1111/imm.13377
Distinct responses of human peripheral blood cells to different misfolded protein oligomers
Abstract
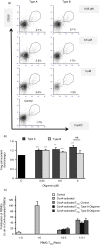
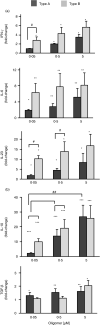
Increasing evidence indicates that peripheral immune cells play a prominent role in neurodegeneration connected to protein misfolding, which are associated with formation of aberrant aggregates, including soluble protein misfolded oligomers. The precise links, however, between the physicochemical features of diverse oligomers and their effects on the immune system, particularly on adaptive immunity, remain currently unexplored, due partly to the transient and heterogeneous nature of the oligomers themselves. To overcome these limitations, we took advantage of two stable and well-characterized types of model oligomers (A and B), formed by HypF-N bacterial protein, type B oligomers displaying lower solvent-exposed hydrophobicity. Exposure to oligomers of human peripheral blood mononuclear cells (PBMCs) revealed differential effects, with type B, but not type A, oligomers leading to a reduction in CD4+ cells. Type A oligomers promoted enhanced differentiation towards CD4+ CD25High FoxP3+ Tregs and displayed a higher suppressive effect on lymphocyte proliferation than Tregs treated with oligomers B or untreated cells. Moreover, our results reveal Th1 and Th17 lymphocyte differentiation mediated by type A oligomers and a differential balance of TGF-β, IL-6, IL-23, IFN-γ and IL-10 mediators. These results indicate that type B oligomers recapitulate some of the biological responses associated with Parkinson's disease in peripheral immunocompetent cells, while type A oligomers resemble responses associated with Alzheimer's disease. We anticipate that further studies characterizing the differential effects of protein misfolded oligomers on the peripheral immune system may lead to the development of blood-based diagnostics, which could report on the type and properties of oligomers present in patients.
Keywords: Th1/Th17; Tregs; immunoregulation; neurodegeneration; peripheral immunity; protein misfolding diseases.
© 2021 The Authors. Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors state no conflict of interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

