Tumor-stroma ratio is associated with Miller-Payne score and pathological response to neoadjuvant chemotherapy in HER2-negative early breast cancer
- PMID: 34043821
- PMCID: PMC8362217
- DOI: 10.1002/ijc.33700
Tumor-stroma ratio is associated with Miller-Payne score and pathological response to neoadjuvant chemotherapy in HER2-negative early breast cancer
Abstract
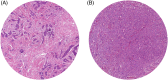
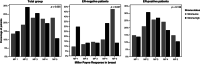
The tumor-stroma ratio (TSR) has proven to be a strong prognostic factor in breast cancer, demonstrating better survival for patients with stroma-low tumors. Since the role of the TSR as a predictive marker for neoadjuvant chemotherapy outcome is yet unknown, this association was evaluated for HER2-negative breast cancer in the prospective DIRECT and NEOZOTAC trials. The TSR was assessed on 375 hematoxylin and eosin-stained sections of pre-treatment biopsies. Associations between the TSR and chemotherapy response according to the Miller-Payne (MP) grading system, and between the TSR and pathological response were examined using Pearson's chi-square, Cochran-Armitage test for trend and regression analyses. A stroma-low tumor prior to neoadjuvant chemotherapy was significantly associated with a higher MP score (P = .005). This relationship remained significant in the estrogen receptor (ER)-negative subgroup (P = .047). The univariable odds ratio (OR) of a stroma-low tumor on pathological complete response (pCR) was 2.46 (95% CI 1.34-4.51, P = .004), which attenuated to 1.90 (95% CI 0.85-4.25, P = .119) after adjustment for relevant prognostic factors. Subgroup analyses revealed an OR of 5.91 in univariable analyses for ER-negativity (95% CI 1.19-29.48, P = .030) and 1.48 for ER-positivity (95% CI 0.73-3.01, P = .281). In conclusion, a low amount of stroma on pre-treatment biopsies is associated with a higher MP score and pCR rate. Therefore, the TSR is a promising biomarker in predicting neoadjuvant treatment outcome. Incorporating this parameter in routine pathological diagnostics could be worthwhile to prevent overtreatment and undertreatment.
Keywords: Miller-Payne; breast cancer; neoadjuvant; pathological response; tumor-stroma ratio.
© 2021 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

Similar articles
-
The potential prognostic value of connexin 26 and 46 expression in neoadjuvant-treated breast cancer.BMC Cancer. 2013 Feb 2;13:50. doi: 10.1186/1471-2407-13-50. BMC Cancer. 2013. PMID: 23374644 Free PMC article.
-
Circulating tumour cell enumeration does not correlate with Miller-Payne grade in a cohort of breast cancer patients undergoing neoadjuvant chemotherapy.Breast Cancer Res Treat. 2020 Jun;181(3):571-580. doi: 10.1007/s10549-020-05658-7. Epub 2020 May 6. Breast Cancer Res Treat. 2020. PMID: 32378053 Free PMC article.
-
IHC4 and COMBINE scores for enhanced prognostic stratification in HR+/HER2- breast cancer patients after neoadjuvant chemotherapy.Breast Cancer Res Treat. 2025 Jun;211(2):307-319. doi: 10.1007/s10549-025-07645-2. Epub 2025 Feb 15. Breast Cancer Res Treat. 2025. PMID: 39954110 Free PMC article.
-
Clinical and pathological response to neoadjuvant chemotherapy based on primary tumor reduction is correlated to survival in hormone receptor-positive but not hormone receptor-negative locally advanced breast cancer.Ann Surg Oncol. 2015 Jan;22(1):32-9. doi: 10.1245/s10434-014-3894-0. Epub 2014 Jul 11. Ann Surg Oncol. 2015. PMID: 25012266
-
Research advances in evaluation methods for neoadjuvant therapy of tumors.Front Oncol. 2025 May 22;15:1580360. doi: 10.3389/fonc.2025.1580360. eCollection 2025. Front Oncol. 2025. PMID: 40475013 Free PMC article. Review.
Cited by
-
Targeting the tumor stroma for cancer therapy.Mol Cancer. 2022 Nov 2;21(1):208. doi: 10.1186/s12943-022-01670-1. Mol Cancer. 2022. PMID: 36324128 Free PMC article. Review.
-
Predicting neoadjuvant chemotherapy benefit using deep learning from stromal histology in breast cancer.NPJ Breast Cancer. 2022 Nov 22;8(1):124. doi: 10.1038/s41523-022-00491-1. NPJ Breast Cancer. 2022. PMID: 36418332 Free PMC article.
-
Comprehensive Analysis of the Effects of Genetic Ancestry and Genetic Characteristics on the Clinical Evolution of Oral Squamous Cell Carcinoma.Front Cell Dev Biol. 2021 Dec 7;9:678464. doi: 10.3389/fcell.2021.678464. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34950653 Free PMC article.
-
Tumor-stroma type and tumor-stroma ratio predict neoadjuvant chemotherapy response in breast cancer.Rev Assoc Med Bras (1992). 2025 Mar 31;71(2):e20241225. doi: 10.1590/1806-9282.20241225. eCollection 2025. Rev Assoc Med Bras (1992). 2025. PMID: 40172391 Free PMC article.
-
Artificial Intelligence-Based Pathology to Assist Prediction of Neoadjuvant Therapy Responses for Breast Cancer.Cancer Med. 2025 Aug;14(15):e71132. doi: 10.1002/cam4.71132. Cancer Med. 2025. PMID: 40762329 Free PMC article. Review.
References
-
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356‐387. - PubMed
-
- Kaufmann M, von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18(12):1927‐1934. - PubMed
-
- Mieog J, Van der Hage J, CJH VDV. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94(10):1189‐1200. - PubMed
-
- Spronk PER, Volders JH, van den Tol P, Smorenburg CH, Vrancken PM. Breast conserving therapy after neoadjuvant chemotherapy; data from the Dutch breast cancer audit. Eur J Surg Oncol. 2019;45(2):110‐117. - PubMed
-
- Cain H, Macpherson IR, Beresford M, Pinder SE, Pong J, Dixon JM. Neoadjuvant therapy in early breast cancer: treatment considerations and common debates in practice. Clin Oncol (R Coll Radiol). 2017;29(10):642‐652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

