Strategies for Covalent Labeling of Long RNAs
- PMID: 34043861
- PMCID: PMC8518768
- DOI: 10.1002/cbic.202100161
Strategies for Covalent Labeling of Long RNAs
Abstract
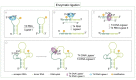
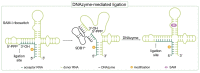
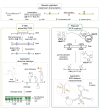
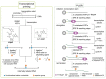
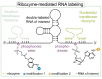
The introduction of chemical modifications into long RNA molecules at specific positions for visualization, biophysical investigations, diagnostic and therapeutic applications still remains challenging. In this review, we present recent approaches for covalent internal labeling of long RNAs. Topics included are the assembly of large modified RNAs via enzymatic ligation of short synthetic oligonucleotides and synthetic biology approaches preparing site-specifically modified RNAs via in vitro transcription using an expanded genetic alphabet. Moreover, recent approaches to employ deoxyribozymes (DNAzymes) and ribozymes for RNA labeling and RNA methyltransferase based labeling strategies are presented. We discuss the potentials and limits of the individual methods, their applicability for RNAs with several hundred to thousands of nucleotides in length and indicate future directions in the field.
Keywords: RNA labeling; bioorthogonal labeling; click chemistry; lncRNA; ribozymes.
© 2021 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- None
-
- Mattick J. S., Makunin I. V., Hum. Mol. Genet. 2006, 15 Spec No 1, R17–29; - PubMed
-
- Kapranov P., Cheng J., Dike S., Nix D. A., Duttagupta R., Willingham A. T., Stadler P. F., Hertel J., Hackermüller J., Hofacker I. L., Bell I, Cheung E., Drenkow J., Dumais E., Patel S., Helt G., Ganesh M., Ghosh S., Piccolboni A., Sementchenko V., Tammana H., Gingeras T. R., Science 2007, 316, 1484–1488. - PubMed
-
- Kirk S., Bioorg. Med. Chem. 2001, 9, 2295–2301. - PubMed
-
- Höbartner C., Sicoli G., Wachowius F., Gophane D. B., Sigurdsson S. T., J. Org. Chem. 2012, 77, 7749–7754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

