Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism
- PMID: 34043943
- PMCID: PMC8280952
- DOI: 10.1016/j.cmet.2021.05.002
Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism
Abstract
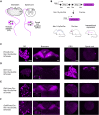
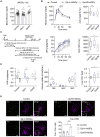
Sensory neurons relay gut-derived signals to the brain, yet the molecular and functional organization of distinct populations remains unclear. Here, we employed intersectional genetic manipulations to probe the feeding and glucoregulatory function of distinct sensory neurons. We reconstruct the gut innervation patterns of numerous molecularly defined vagal and spinal afferents and identify their downstream brain targets. Bidirectional chemogenetic manipulations, coupled with behavioral and circuit mapping analysis, demonstrated that gut-innervating, glucagon-like peptide 1 receptor (GLP1R)-expressing vagal afferents relay anorexigenic signals to parabrachial nucleus neurons that control meal termination. Moreover, GLP1R vagal afferent activation improves glucose tolerance, and their inhibition elevates blood glucose levels independent of food intake. In contrast, gut-innervating, GPR65-expressing vagal afferent stimulation increases hepatic glucose production and activates parabrachial neurons that control normoglycemia, but they are dispensable for feeding regulation. Thus, distinct gut-innervating sensory neurons differentially control feeding and glucoregulatory neurocircuits and may provide specific targets for metabolic control.
Keywords: Dre-recombinase; appetite; chemogenetics; dorsal root ganglion; glucose metabolism; gut-brain axis; intersectional genetics; nodose ganglion; sensory neurons; vagus nerve.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Comment in
-
Mapping neuron functions in the gut-brain axis.Nat Rev Endocrinol. 2021 Aug;17(8):448. doi: 10.1038/s41574-021-00519-9. Nat Rev Endocrinol. 2021. PMID: 34099905 No abstract available.
References
-
- Akopian A.N., Sivilotti L., Wood J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

