Systems biology evaluation of refractory Clostridioides difficile infection including multiple failures of fecal microbiota transplantation
- PMID: 34044101
- PMCID: PMC8384661
- DOI: 10.1016/j.anaerobe.2021.102387
Systems biology evaluation of refractory Clostridioides difficile infection including multiple failures of fecal microbiota transplantation
Abstract
Background: Fecal microbiota transplantation (FMT) aims to cure Clostridioides difficile infection (CDI) through reestablishing a healthy microbiome and restoring colonization resistance. Although often effective after one infusion, patients with continued microbiome disruptions may require multiple FMTs. In this N-of-1 study, we use a systems biology approach to evaluate CDI in a patient receiving chronic suppressive antibiotics with four failed FMTs over two years.
Methods: Seven stool samples were obtained between 2016-18 while the patient underwent five FMTs. Stool samples were cultured for C. difficile and underwent microbial characterization and functional gene analysis using shotgun metagenomics. C. difficile isolates were characterized through ribotyping, whole genome sequencing, metabolic pathway analysis, and minimum inhibitory concentration (MIC) determinations.
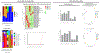
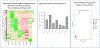
Results: Growing ten strains from each sample, the index and first four recurrent cultures were single strain ribotype F078-126, the fifth was a mixed culture of ribotypes F002 and F054, and the final culture was ribotype F002. One single nucleotide polymorphism (SNP) variant was identified in the RNA polymerase (RNAP) β-subunit RpoB in the final isolated F078-126 strain when compared to previous F078-126 isolates. This SNV was associated with metabolic shifts but phenotypic differences in fidaxomicin MIC were not observed. Microbiome differences were observed over time during vancomycin therapy and after failed FMTs.
Conclusion: This study highlights the importance of antimicrobial stewardship in patients receiving FMT. Continued antibiotics play a destructive role on a transplanted microbiome and applies selection pressure for resistance to the few antibiotics available to treat CDI.
Keywords: Clostridium difficile; Metabolic analysis; Microbiome; N-of-1 studies; Shotgun sequencing.
Crown Copyright © 2021. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest All authors state no conflicts of interest.
Figures
Similar articles
-
The presence of Clostridioides difficile in faeces before and after faecal microbiota transplantation and its relation with recurrent C. difficile infection and the gut microbiota in a Dutch cohort.Clin Microbiol Infect. 2025 Apr;31(4):568-574. doi: 10.1016/j.cmi.2024.12.003. Epub 2024 Dec 9. Clin Microbiol Infect. 2025. PMID: 39662821
-
Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease.Microbiome. 2017 May 15;5(1):55. doi: 10.1186/s40168-017-0269-3. Microbiome. 2017. PMID: 28506317 Free PMC article.
-
Gram-Negative Taxa and Antimicrobial Susceptibility after Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection.mSphere. 2020 Oct 14;5(5):e00853-20. doi: 10.1128/mSphere.00853-20. mSphere. 2020. PMID: 33055258 Free PMC article.
-
Microbiota-based Therapies Clostridioides difficile infection that is refractory to antibiotic therapy.Transl Res. 2021 Apr;230:197-207. doi: 10.1016/j.trsl.2020.11.013. Epub 2020 Dec 2. Transl Res. 2021. PMID: 33278650 Review.
-
The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection.Therap Adv Gastroenterol. 2022 Nov 18;15:17562848221134396. doi: 10.1177/17562848221134396. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 36425405 Free PMC article. Review.
Cited by
-
Computational approaches to understanding Clostridioides difficile metabolism and virulence.Curr Opin Microbiol. 2022 Feb;65:108-115. doi: 10.1016/j.mib.2021.11.002. Epub 2021 Nov 25. Curr Opin Microbiol. 2022. PMID: 34839237 Free PMC article. Review.
-
Fecal Pharmacokinetics and Gut Microbiome Effects of Oral Omadacycline Versus Vancomycin in Healthy Volunteers.J Infect Dis. 2024 Jan 12;229(1):273-281. doi: 10.1093/infdis/jiad537. J Infect Dis. 2024. PMID: 38051631 Free PMC article. Clinical Trial.
-
Genetic Mechanisms of Vancomycin Resistance in Clostridioides difficile: A Systematic Review.Antibiotics (Basel). 2022 Feb 16;11(2):258. doi: 10.3390/antibiotics11020258. Antibiotics (Basel). 2022. PMID: 35203860 Free PMC article. Review.
-
A Vancomycin HPLC Assay for Use in Gut Microbiome Research.Microbiol Spectr. 2022 Jun 29;10(3):e0168821. doi: 10.1128/spectrum.01688-21. Epub 2022 May 10. Microbiol Spectr. 2022. PMID: 35536037 Free PMC article.
-
Efficacy, Safety, Pharmacokinetics, and Microbiome Changes of Ibezapolstat in Adults with Clostridioides difficile Infection: A Phase 2a Multicenter Clinical Trial.Clin Infect Dis. 2022 Sep 30;75(7):1164-1170. doi: 10.1093/cid/ciac096. Clin Infect Dis. 2022. PMID: 35134880 Free PMC article. Clinical Trial.
References
-
- McDonald LC, Gerding DN, Johnson S, et al.Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66(7):987–994. DOI: 10.1093/cid/ciy149. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases