Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street
- PMID: 34044180
- PMCID: PMC8513145
- DOI: 10.1016/j.molmet.2021.101261
Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street
Abstract
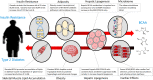
Background: A strong association of obesity and insulin resistance with increased circulating levels of branched-chain and aromatic amino acids and decreased glycine levels has been recognized in human subjects for decades.
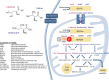
Scope of review: More recently, human metabolomics and genetic studies have confirmed and expanded upon these observations, accompanied by a surge in preclinical studies that have identified mechanisms involved in the perturbation of amino acid homeostasis- how these events are connected to dysregulated glucose and lipid metabolism, and how elevations in branched-chain amino acids (BCAA) may participate in the development of insulin resistance, type 2 diabetes (T2D), and other cardiometabolic diseases and conditions.
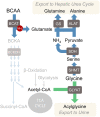
Major conclusions: In human cohorts, BCAA and related metabolites are now well established as among the strongest biomarkers of obesity, insulin resistance, T2D, and cardiovascular diseases. Lowering of BCAA and branched-chain ketoacid (BCKA) levels by feeding BCAA-restricted diet or by the activation of the rate-limiting enzyme in BCAA catabolism, branched-chain ketoacid dehydrogenase (BCKDH), in rodent models of obesity have clear salutary effects on glucose and lipid homeostasis, but BCAA restriction has more modest effects in short-term studies in human T2D subjects. Feeding of rats with diets enriched in sucrose or fructose result in the induction of the ChREBP transcription factor in the liver to increase expression of the BCKDH kinase (BDK) and suppress the expression of its phosphatase (PPM1K) resulting in the inactivation of BCKDH and activation of the key lipogenic enzyme ATP-citrate lyase (ACLY). These and other emergent links between BCAA, glucose, and lipid metabolism motivate ongoing studies of possible causal actions of BCAA and related metabolites in the development of cardiometabolic diseases.
Keywords: Branched-chain amino acids; Insulin resistance; Lipogenesis; Metabolic diseases; Nutrition.
Copyright © 2021 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures
References
-
- Felig P., Marliss E., Cahill G.F. Plasma amino acid levels and insulin secretion in obesity. New England Journal of Medicine. 1969;281:811–816. - PubMed
-
- Lynch B.C.K.A., She P., Van Horn C., Reid T., Hutson S.M., Cooney R.N. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. American Journal of Physiology. Endocrinology and Metabolism. 2007;293:E1552–E1563. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

