Amyotrophic lateral sclerosis alters the metabolic aging profile in patient derived fibroblasts
- PMID: 34044197
- PMCID: PMC8346650
- DOI: 10.1016/j.neurobiolaging.2021.04.013
Amyotrophic lateral sclerosis alters the metabolic aging profile in patient derived fibroblasts
Abstract
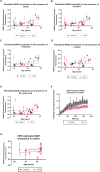
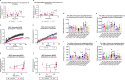
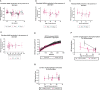
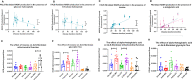
Aging is a major risk factor for neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS). As metabolic alterations are a hallmark of aging and have previously been observed in ALS, it is important to examine the effect of aging in the context of ALS metabolic function. Here, using a newly established phenotypic metabolic approach, we examined the effect of aging on the metabolic profile of fibroblasts derived from ALS cases compared to controls. We found that ALS fibroblasts have an altered metabolic profile, which is influenced by age. In control cases, we found significant increases with age in NADH metabolism in the presence of several metabolites including lactic acid, trehalose, uridine and fructose, which was not recapitulated in ALS cases. Conversely, we found a reduction of NADH metabolism with age of biopsy, age of onset and age of death in the presence of glycogen in the ALS cohort. Furthermore, we found that NADH production correlated with disease progression rates in relation to a number of metabolites including inosine and α-ketoglutaric acid. Inosine or α-ketoglutaric acid supplementation in ALS fibroblasts was bioenergetically favourable. Overall, we found aging related defects in energy substrates that feed carbon into glycolysis at various points as well as the tricarboxylic acid (TCA) cycle in ALS fibroblasts, which was validated in induced neuronal progenitor cell derived iAstrocytes. Our results suggest that supplementing those pathways may protect against age related metabolic dysfunction in ALS.
Keywords: Aging, ALS; Fibroblasts; Metabolism.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors can state there is no conflict of interest associated with this work.
Figures

References
-
- Aguib Y., Heiseke A., Gilch S., Riemer C., Baier M., Schatzl H.M., Ertmer A. Autophagy induction by trehalose counteracts cellular prion infection. Autophagy. 2009;5(3):361–369. - PubMed
-
- Ajroud-Driss S., Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS) Biochim. Biophys. Acta. 2015;1852(4):679–684. - PubMed
-
- Akao T., Yoshino T., Kobashi K., Hattori M. Evaluation of salicin as an antipyretic prodrug that does not cause gastric injury. Planta Med. 2002;68(8):714–718. - PubMed
-
- Allen S.P., Duffy L.M., Shaw P.J., Grierson A.J. Altered age-related changes in bioenergetic properties and mitochondrial morphology in fibroblasts from sporadic amyotrophic lateral sclerosis patients. Neurobiol. Aging. 2015;36(10):2893–2903. - PubMed
-
- Allen S.P., Hall B., Castelli L.M., Francis L., Woof R., Siskos A.P., Kouloura E., Gray E., Thompson A.G., Talbot K., Higginbottom A., Myszczynska M., Allen C.F., Stopford M.J., Hemingway J., Bauer C.S., Webster C.P., De Vos K.J., Turner M.R., Keun H.C., Hautbergue G.M., Ferraiuolo L., Shaw P.J. Astrocyte adenosine deaminase loss increases motor neuron toxicity in amyotrophic lateral sclerosis. Brain. 2019;142(3):586–605. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

