Follow-up definitions in clinical orthopaedic research : a systematic review
- PMID: 34044582
- PMCID: PMC8168549
- DOI: 10.1302/2633-1462.25.BJO-2021-0007.R1
Follow-up definitions in clinical orthopaedic research : a systematic review
Abstract
Aims: The follow-up interval of a study represents an important aspect that is frequently mentioned in the title of the manuscript. Authors arbitrarily define whether the follow-up of their study is short-, mid-, or long-term. There is no clear consensus in that regard and definitions show a large range of variation. It was therefore the aim of this study to systematically identify clinical research published in high-impact orthopaedic journals in the last five years and extract follow-up information to deduce corresponding evidence-based definitions of short-, mid-, and long-term follow-up.
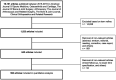
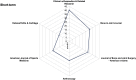
Methods: A systematic literature search was performed to identify papers published in the six highest ranked orthopaedic journals during the years 2015 to 2019. Follow-up intervals were analyzed. Each article was assigned to a corresponding subspecialty field: sports traumatology, knee arthroplasty and reconstruction, hip-preserving surgery, hip arthroplasty, shoulder and elbow arthroplasty, hand and wrist, foot and ankle, paediatric orthopaedics, orthopaedic trauma, spine, and tumour. Mean follow-up data were tabulated for the corresponding subspecialty fields. Comparison between means was conducted using analysis of variance.
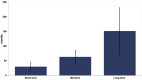
Results: Of 16,161 published articles, 590 met the inclusion criteria. Of these, 321 were of level IV evidence, 176 level III, 53 level II, and 40 level I. Considering all included articles, a long-term study published in the included high impact journals had a mean follow-up of 151.6 months, a mid-term study of 63.5 months, and a short-term study of 30.0 months.
Conclusion: The results of this study provide evidence-based definitions for orthopaedic follow-up intervals that should provide a citable standard for the planning of clinical studies. A minimum mean follow-up of a short-term study should be 30 months (2.5 years), while a mid-term study should aim for a mean follow-up of 60 months (five years), and a long-term study should aim for a mean of 150 months (12.5 years). Level of Evidence: Level I. Cite this article: Bone Jt Open 2021;2(5):344-350.
Keywords: Clinical study; Long-term; Mid-term; Outcome; Short-term; Study design.
Figures





References
-
- Young JM, Solomon MJ. Improving the evidence base in surgery: sources of bias in surgical studies. ANZ J Surg. 2003;73(7):504–506. - PubMed
-
- Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM levels of evidence (introductory document). Oxford Center for Evidence-Based Medicine. 2011. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-e... (date last accessed 25 May 2021).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

