Restructured Mitochondrial-Nuclear Interaction in Plasmodium falciparum Dormancy and Persister Survival after Artemisinin Exposure
- PMID: 34044591
- PMCID: PMC8262848
- DOI: 10.1128/mBio.00753-21
Restructured Mitochondrial-Nuclear Interaction in Plasmodium falciparum Dormancy and Persister Survival after Artemisinin Exposure
Abstract
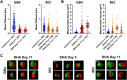
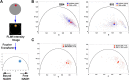
Artemisinin and its semisynthetic derivatives (ART) are fast acting, potent antimalarials; however, their use in malaria treatment is frequently confounded by recrudescences from bloodstream Plasmodium parasites that enter into and later reactivate from a dormant persister state. Here, we provide evidence that the mitochondria of dihydroartemisinin (DHA)-exposed persisters are dramatically altered and enlarged relative to the mitochondria of young, actively replicating ring forms. Restructured mitochondrial-nuclear associations and an altered metabolic state are consistent with stress from reactive oxygen species. New contacts between the mitochondria and nuclei may support communication pathways of mitochondrial retrograde signaling, resulting in transcriptional changes in the nucleus as a survival response. Further characterization of the organelle communication and metabolic dependencies of persisters may suggest strategies to combat recrudescences of malaria after treatment. IMPORTANCE The major first-line treatment for malaria, especially the deadliest form caused by Plasmodium falciparum, is combination therapy with an artemisinin-based drug (ART) plus a partner drug to assure complete cure. Without an effective partner drug, ART administration alone can fail because of the ability of small populations of blood-stage malaria parasites to enter into a dormant state and survive repeated treatments for a week or more. Understanding the nature of parasites in dormancy (persisters) and their ability to wake and reestablish actively propagating parasitemias (recrudesce) after ART exposure may suggest strategies to improve treatment outcomes and counter the threats posed by parasites that develop resistance to partner drugs. Here, we show that persisters have dramatically altered mitochondria and mitochondrial-nuclear interactions associated with features of metabolic quiescence. Restructured associations between the mitochondria and nuclei may support signaling pathways that enable the ART survival responses of dormancy.
Keywords: Airyscan microscopy; artemisinin-based combination therapy; drug resistance; fluorescence lifetime imaging; malaria; mitochondrial retrograde response.
Figures


References
-
- World Health Organization. 2015. Guidelines for the treatment of malaria, 3rd ed. World Health Organization, Geneva, Switzerland.
-
- Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, Agbenyega T, Nguah SB, Evans J, Gesase S, Kahabuka C, Mtove G, Nadjm B, Deen J, Mwanga-Amumpaire J, Nansumba M, Karema C, Umulisa N, Uwimana A, Mokuolu OA, Adedoyin OT, Johnson WB, Tshefu AK, Onyamboko MA, Sakulthaew T, Ngum WP, Silamut K, Stepniewska K, Woodrow CJ, Bethell D, Wills B, Oneko M, Peto TE, von Seidlein L, Day NP, White NJ. 2010. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376:1647–1657. doi:10.1016/S0140-6736(10)61924-1. - DOI - PMC - PubMed
-
- Hoshen MB, Na-Bangchang K, Stein WD, Ginsburg H. 2000. Mathematical modelling of the chemotherapy of Plasmodium falciparum malaria with artesunate: postulation of 'dormancy', a partial cytostatic effect of the drug, and its implication for treatment regimens. Parasitology 121:237–246. doi:10.1017/S0031182099006332. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
