Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis
- PMID: 34044777
- PMCID: PMC8159019
- DOI: 10.1186/s12879-021-06164-x
Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis
Abstract
Background: Favipiravir possesses high utility for treating patients with COVID-19. However, research examining the efficacy and safety of favipiravir for patients with COVID-19 is limited.
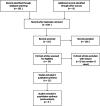
Methods: We conducted a systematic review of published studies reporting the efficacy of favipiravir against COVID-19. Two investigators independently searched PubMed, the Cochrane Database of Systematic Reviews, MedRxiv, and ClinicalTrials.gov (inception to September 2020) to identify eligible studies. A meta-analysis was performed to measure viral clearance and clinical improvement as the primary outcomes.
Results: Among 11 eligible studies, 5 included a comparator group. Comparing to the comparator group, the favipiravir group exhibited significantly better viral clearance on day 7 after the initiation of treatment (odds ratio [OR] = 2.49, 95% confidence interval [CI] = 1.19-5.22), whereas no difference was noted on day 14 (OR = 2.19, 95% CI = 0.69-6.95). Although clinical improvement was significantly better in the favipiravir group on both days 7 and 14, the improvement was better on day 14 (OR = 3.03, 95% CI = 1.17-7.80) than on day 7 (OR = 1.60, 95% CI = 1.03-2.49). The estimated proportions of patients with viral clearance in the favipiravir arm on days 7 and 14 were 65.42 and 88.9%, respectively, versus 43.42 and 78.79%, respectively, in the comparator group. The estimated proportions of patients with clinical improvement on days 7 and 14 in the favipiravir group were 54.33 and 84.63%, respectively, compared with 34.40 and 65.77%, respectively, in the comparator group.
Conclusions: Favipiravir induces viral clearance by 7 days and contributes to clinical improvement within 14 days. The results indicated that favipiravir has strong possibility for treating COVID-19, especially in patients with mild-to-moderate illness. Additional well-designed studies, including examinations of the dose and duration of treatment, are crucial for reaching definitive conclusions.
Keywords: COVID-19; Clinical improvement; Favipiravir; SARS-CoV-2; Viral clearance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. Available at https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re... (Accessed April 5, 2020).
-
- World Health Organization. Weekly epidemiological update-1 December 2020. Available at https://www.who.int/publications/m/item/weekly-epidemiological-update%2D... (Accessed December 5, 2020).
-
- FUJIFILM Toyama Chemical Co., Ltd. Drug Interview form. Avigan® http://fftc.fujifilm.co.jp/med/abigan/pack/pdf/abigan_if_01.pdf. Accessed 5 Apr 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

